CIBINQO
(abrocitinib)
Find CIBINQO medical information:
Find CIBINQO medical information:
CIBINQO Quick Finder
Highlights
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CIBINQO safely and effectively. See full prescribing information for CIBINQO. CIBINQO® (abrocitinib) tablets, for oral use Initial U.S. Approval: 2022 WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), and THROMBOSISSee full prescribing information for complete boxed warning.
RECENT MAJOR CHANGESINDICATIONS AND USAGECIBINQO is a Janus kinase (JAK) inhibitor indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable. (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSCIBINQO Tablets: 50 mg, 100 mg, and 200 mg (3) CONTRAINDICATIONSAntiplatelet therapies except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment. (4) WARNINGS AND PRECAUTIONSADVERSE REACTIONSMost common adverse events (≥1% with CIBINQO 100 mg) are nasopharyngitis, nausea, headache, herpes simplex, increased blood creatine phosphokinase, dizziness, urinary tract infection, fatigue, acne, vomiting, impetigo, oropharyngeal pain, hypertension, influenza, gastroenteritis, and dermatitis contact. (6.1) Most common adverse reactions (≥1% with CIBINQO 200 mg and greater than CIBINQO 100 mg) are nausea, headache, herpes simplex, increased blood creatine kinase, dizziness, urinary tract infection, acne, vomiting, gastroenteritis, upper abdominal pain, abdominal discomfort, herpes zoster, and thrombocytopenia. (6.1) DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 12/2023 |
Boxed Warning
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, and THROMBOSIS
Serious Infections
Patients treated with CIBINQO may be at increased risk for developing serious infections that may lead to hospitalization or death. The most frequent serious infections reported with CIBINQO were herpes simplex, herpes zoster, and pneumonia [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
If a serious or opportunistic infection develops, discontinue CIBINQO and control the infection.
Reported infections from Janus kinase (JAK) inhibitors used to treat inflammatory conditions:
- •
- Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative latent TB test.
- •
- Invasive fungal infections, including cryptococcosis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
- •
- Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
Avoid use of CIBINQO in patients with an active, serious infection including localized infections. The risks and benefits of treatment with CIBINQO should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIBINQO, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy [see Warnings and Precautions (5.1)].
Mortality
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another JAK inhibitor to TNF blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor. CIBINQO is not approved for use in RA patients [see Warnings and Precautions (5.2)].
Malignancies
Malignancies were reported in patients treated with CIBINQO. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with another JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk [see Warnings and Precautions (5.3)].
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in patients treated with CIBINQO. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke [see Warnings and Precautions (5.4)].
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with CIBINQO. Thrombosis, including PE, DVT, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid CIBINQO in patients at risk. If symptoms of thrombosis occur, discontinue CIBINQO and treat appropriately [see Warnings and Precautions (5.5)].
Indications and Usage
1 INDICATIONS AND USAGE
CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Testing, Evaluations, and Procedures Prior to Treatment Initiation
Perform the following tests and evaluations prior to CIBINQO initiation:
- •
- Tuberculosis (TB) infection evaluation – CIBINQO initiation is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk for TB, start preventive therapy for latent TB prior to initiation of CIBINQO [see Warnings and Precautions (5.1)].
- •
- Viral hepatitis screening in accordance with clinical guidelines – CIBINQO initiation is not recommended in patients with active hepatitis B or hepatitis C [see Warnings and Precautions (5.1)].
- •
- A complete blood count (CBC) – CIBINQO initiation is not recommended in patients with a platelet count <150,000/mm3, an absolute lymphocyte count <500/mm3, an absolute neutrophil count <1,000/mm3, or a hemoglobin value <8 g/dL [see Warnings and Precautions (5.6)].
Complete any necessary immunizations, including herpes zoster vaccinations, in agreement with current immunization guidelines prior to CIBINQO initiation [see Warnings and Precautions (5.7)].
2.2 Recommended Dosage
The recommended dose is 100 mg once daily. If an adequate response is not achieved with CIBINQO 100 mg once daily, consider increasing the dosage to 200 mg once daily.
Discontinue CIBINQO if an adequate response is not achieved with 200 mg once daily.
Use the lowest efficacious dose to maintain response.
CIBINQO can be used with or without topical corticosteroids.
If a dose is missed, administer the dose as soon as possible unless it is less than 12 hours before the next dose, in which case skip the missed dose. Thereafter, resume dosing at the regular scheduled time.
2.3 Recommended Dosage in Patients with Renal Impairment or Hepatic Impairment
Renal Impairment
CIBINQO dosage recommendations for patients with renal impairment are provided in Table 1 [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. In patients with mild and moderate renal impairment, if an adequate response is not achieved with initial dose, the dose of CIBINQO can be doubled [see Dosage and Administration (2.2)].
Renal Impairment Stage | Estimated Glomerular Filtration (eGFR)* | Dosage |
Mild | 60 – 89 mL/minute | CIBINQO 100 mg once daily |
Moderate | 30 – 59 mL/minute | CIBINQO 50 mg once daily |
Severe† | 15 – 29 mL/minute | Not recommended for use |
End-Stage Renal Disease† (ESRD) | <15 mL/minute | |
Hepatic Impairment
CIBINQO is not recommended for use in patients with severe hepatic impairment [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.4 Recommended Dosage in CYP2C19 Poor Metabolizers
In patients who are known or suspected to be CYP2C19 poor metabolizers, the recommended dosage of CIBINQO is 50 mg once daily [see Use in Specific Populations (8.8) and Clinical Pharmacology (12.5)]. If an adequate response is not achieved with CIBINQO 50 mg once daily, consider increasing the dosage to 100 mg once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
2.5 Dosage Modifications due to Strong Inhibitors
In patients taking strong inhibitors of cytochrome P450 (CYP) 2C19, reduce the dosage to 50 mg once daily [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. If an adequate response is not achieved with CIBINQO 50 mg daily, consider increasing the dosage to 100 mg once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
2.6 Treatment Discontinuation due to Serious Infections or Hematologic Adverse Reactions
Serious or Opportunistic Infections
If a patient develops a serious or opportunistic infection, discontinue CIBINQO and control the infection. The risks and benefits of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO [see Warnings and Precautions (5.1)].
Hematologic Abnormalities
Recommendations for CIBINQO discontinuation for laboratory abnormalities are summarized in Table 2.
| Abbreviations: ALC=absolute lymphocyte count; ANC=absolute neutrophil count; CBC=complete blood count; Hb=hemoglobin | |
Laboratory Measure | Recommendation |
Platelet Count <50,000/mm3 | Discontinue CIBINQO and follow with CBC until >100,000/mm3 |
ALC <500/mm3 | Treatment should be temporarily discontinued if ALC is less than 500 cells/mm3 and may be restarted once ALC return above this value |
ANC <1,000/mm3 | Treatment should be temporarily discontinued if ANC is less than 1,000 cells/mm3 and may be restarted once ANC return above this value |
Hb value <8 g/dL | Treatment should be temporarily discontinued if Hb is less than 8 g/dL and may be restarted once Hb return above this value |
CBC evaluations are recommended at baseline, 4 weeks after treatment initiation and 4 weeks after dosage increase of CIBINQO. Laboratory evaluations may be extended for patients on chronic CIBINQO therapy who develop hematologic abnormalities [see Warnings and Precautions (5.6)].
Dosage Forms and Strengths
3 DOSAGE FORMS AND STRENGTHS
- •
- 50 mg: Pink, oval, film-coated tablet debossed with "PFE" on one side and "ABR 50" on the other.
- •
- 100 mg: Pink, round, film-coated tablet debossed with "PFE" on one side and "ABR 100" on the other.
- •
- 200 mg: Pink, oval, film-coated tablet debossed with "PFE" on one side and "ABR 200" on the other.
Contraindications
4 CONTRAINDICATIONS
CIBINQO is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment [see Warnings and Precautions (5.6), Drug Interactions (7.2), and Clinical Pharmacology (12.2)].
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
The most frequent serious infections reported in clinical studies with CIBINQO for atopic dermatitis were herpes simplex, herpes zoster, and pneumonia [see Adverse Reactions (6.1)]. Serious infections leading to hospitalization or death, including tuberculosis and bacterial, invasive fungal, viral, and other opportunistic infections, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions.
Avoid use of CIBINQO in patients with active, serious infection including localized infections.
Consider the risks and benefits of treatment prior to initiating CIBINQO in patients:
- •
- with chronic or recurrent infection
- •
- who have been exposed to tuberculosis
- •
- with a history of a serious or an opportunistic infection
- •
- who have resided or traveled in areas of endemic tuberculosis or endemic mycoses
- •
- with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with CIBINQO. If a patient develops a serious or opportunistic infection, discontinue CIBINQO. Initiate complete diagnostic testing and appropriate antimicrobial therapy. The risks and benefits of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO.
Tuberculosis
Evaluate and test patients for TB before starting CIBINQO therapy and consider yearly screening for patients in highly endemic areas for TB. CIBINQO is not recommended for use in patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, or for patients with a negative test for latent TB but who are at high risk for TB infection, start preventive therapy for latent TB prior to initiation of CIBINQO. Monitor patients for the development of signs and symptoms of TB, including patients who were tested negative for latent TB infection prior to initiating therapy.
Viral Reactivation
Viral reactivation, including herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical trials with CIBINQO [see Adverse Reactions (6.1)]. If a patient develops herpes zoster, consider interrupting CIBINQO until the episode resolves.
Hepatitis B virus (HBV) reactivation has been reported in patients receiving JAK inhibitors. Perform viral hepatitis screening in accordance with clinical guidelines before starting therapy and monitor for reactivation during therapy with CIBINQO. CIBINQO is not recommended for use in patients with active hepatitis B or hepatitis C [see Clinical Pharmacology (12.3)]. Monitor patients with inactive HBV for expression of HBV DNA during therapy with CIBINQO. If HBV DNA is detected during therapy with CIBINQO, consult a liver specialist.
5.2 Mortality
In a large, randomized, postmarketing safety trial of another JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in subjects treated with the JAK inhibitor compared with TNF blockers. CIBINQO is not approved for use in RA.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO.
5.3 Malignancy and Lymphoproliferative Disorders
Malignancies, including non-melanoma skin cancer (NMSC), were observed in clinical trials with CIBINQO for atopic dermatitis [see Adverse Reactions (6.1)].
Perform periodic skin examination for patients who are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions. In a large, randomized, postmarketing safety trial of another JAK inhibitor in RA subjects, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed in subjects treated with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. A higher rate of lymphomas was observed in subjects treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this trial, current or past smokers had an additional increased risk of overall malignancies.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
5.4 Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in clinical trials of CIBINQO for atopic dermatitis [see Adverse Reactions (6.1)].
In a large, randomized, postmarketing safety trial of another JAK inhibitor in RA subjects 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
5.5 Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) were observed in subjects receiving CIBINQO in the clinical trials for atopic dermatitis [see Adverse Reactions (6.1)].
Thrombosis, including DVT, PE, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death.
In a large, randomized, postmarketing safety trial of another JAK inhibitor in RA subjects 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. CIBINQO is not approved for use in RA.
Avoid CIBINQO in patients that may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue CIBINQO and evaluate and treat patients appropriately.
5.6 Laboratory Abnormalities
Hematologic Abnormalities
Treatment with CIBINQO was associated with an increased incidence of thrombocytopenia and lymphopenia [see Adverse Reactions (6.1)]. Prior to CIBINQO initiation, perform a CBC [see Dosage and Administration (2.1)]. CBC evaluations are recommended at 4 weeks after initiation and 4 weeks after dose increase of CIBINQO. Discontinuation of CIBINQO therapy is required for certain laboratory abnormalities [see Dosage and Administration (2.6)].
Lipid Elevations
Dose-dependent increase in blood lipid parameters were reported in subjects treated with CIBINQO [see Adverse Reactions (6.1)]. Lipid parameters should be assessed approximately 4 weeks following initiation of CIBINQO therapy and thereafter patients should be managed according to clinical guidelines for hyperlipidemia. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined.
Adverse Reactions
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Serious Infections [see Warnings and Precautions (5.1)]
- •
- Mortality [see Warnings and Precautions (5.2)]
- •
- Malignancy and Lymphoproliferative Disorders [see Warnings and Precautions (5.3)]
- •
- Major Adverse Cardiovascular Events [see Warnings and Precautions (5.4)]
- •
- Thrombosis [see Warnings and Precautions (5.5)]
- •
- Laboratory Abnormalities [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CIBINQO was evaluated in four randomized, placebo-controlled clinical trials (2 monotherapy, 1 combination therapy with topical corticosteroid, and 1 dose-ranging) and one long-term extension trial in subjects with moderate to severe atopic dermatitis (AD). A total of 1623 subjects with moderate to severe atopic dermatitis were treated with CIBINQO in these clinical trials representing 1428 patient-years of exposure. There were 634 subjects with at least 1 year of exposure to CIBINQO.
In the placebo-controlled clinical trials, a total of 1198 subjects were exposed to CIBINQO with 608 subjects receiving CIBINQO 100 mg once daily and 590 subjects receiving CIBINQO 200 mg once daily for up to 16 weeks. The median age of subjects was 33.0 years, 124 subjects (8.1%) were 12 to less than 18 years old and 94 subjects (6.1%) were 65 years of age or older. The majority of subjects were White (68.7%) and male (53.9%).
Adverse reactions occurring at ≥1% in any of the treated groups and at a higher rate than in the placebo group are presented in Table 3. A total of 61 (5.1%) subjects treated with CIBINQO were discontinued from the trials due to adverse reactions. The safety profile of CIBINQO in the monotherapy and the combination trial(s) were similar.
Weeks 0–16 | |||
CIBINQO | CIBINQO | Placebo | |
Nasopharyngitis | 51 (8.7) | 75 (12.4) | 27 (7.9) |
Nausea | 86 (14.5) | 37 (6.0) | 7 (2.1) |
Headache | 46 (7.8) | 36 (6.0) | 12 (3.5) |
Herpes simplex† | 25 (4.2) | 20 (3.3) | 6 (1.8) |
Increased blood creatine phosphokinase | 17 (2.9) | 14 (2.3) | 5 (1.5) |
Dizziness | 17 (2.9) | 11 (1.8) | 3 (0.9) |
Urinary tract infection | 13 (2.2) | 10 (1.7) | 4 (1.2) |
Fatigue | 8 (1.3) | 10 (1.6) | 2 (0.5) |
Acne | 28 (4.7) | 10 (1.6) | 0 (0.0) |
Vomiting | 19 (3.2) | 9 (1.5) | 3 (0.9) |
Impetigo | 3 (0.5) | 9 (1.5) | 1 (0.3) |
Oropharyngeal pain | 6 (1.0) | 8 (1.4) | 2 (0.6) |
Hypertension | 5 (0.8) | 7 (1.2) | 2 (0.7) |
Influenza | 6 (1.1) | 7 (1.2) | 0 (0.0) |
Gastroenteritis | 8 (1.3) | 7 (1.1) | 2 (0.6) |
Dermatitis contact | 3 (0.5) | 6 (1.1) | 1 (0.3) |
Abdominal pain upper | 11 (1.9) | 4 (0.6) | 0 (0.0) |
Abdominal discomfort | 7 (1.2) | 3 (0.5) | 1 (0.3) |
Herpes zoster | 7 (1.2) | 2 (0.3) | 0 (0.0) |
Thrombocytopenia | 9 (1.5) | 0 (0.0) | 0 (0.0) |
Specific Adverse Reactions
Exposure adjusted incidence rates were adjusted by trial size for all the adverse reactions reported in this section.
Overall Infections
In the placebo-controlled trials, for up to 16 weeks, overall infections were reported in 90 subjects (126.8 per 100 patient-years) treated with placebo, 211 subjects (168.8 per 100 patient-years) treated with CIBINQO 100 mg and 204 subjects (159.5 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, overall infections were reported in 427 subjects (91.8 per 100 patient-years) treated with CIBINQO 100 mg and 394 subjects (103.2 per 100 patient-years) treated with CIBINQO 200 mg.
Serious Infections
In the placebo-controlled trials, for up to 16 weeks, serious infections were reported in 2 subjects (2.6 per 100 patient-years) treated with placebo, 6 subjects (3.9 per 100 patient-years) treated with CIBINQO 100 mg, and 2 subjects (1.3 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, serious infections were reported in 18 subjects (2.3 per 100 patient-years) treated with CIBINQO 100 mg and 16 subjects (2.3 per 100 patient-years) treated with CIBINQO 200 mg. The most commonly reported serious infections were herpes simplex, herpes zoster, and pneumonia.
Herpes Zoster
In the placebo-controlled trials, for up to 16 weeks, opportunistic infections were generally cases of multidermatomal cutaneous herpes zoster. Herpes zoster was reported in 0 subjects treated with placebo, 3 subjects (1.9 per 100 patient-years) treated with CIBINQO 100 mg and 8 subjects (5.1 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, herpes zoster was reported in 16 subjects (2.0 per 100 patient-years) treated with CIBINQO 100 mg and 35 subjects (5.2 per 100 patient-years) treated with CIBINQO 200 mg.
Malignancy
In the placebo-controlled trials, for up to 16 weeks, no malignancy was reported in subjects treated with placebo or CIBINQO 100 mg and in 1 patient (0.65 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, malignancy was reported in 4 subjects (0.5 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombosis
In all clinical trials, including the long-term extension trial, pulmonary embolism was reported in 3 subjects (0.4 per 100 patient-years), who were treated with CIBINQO 200 mg. Deep vein thrombosis was reported in 2 subjects (0.3 per 100 patient-years) who were treated with CIBINQO 200 mg. No thrombosis occurred in subjects treated with CIBINQO 100 mg.
Major Adverse Cardiovascular Events
In the placebo-controlled trials, for up to 16 weeks, major adverse cardiovascular event (MACE) was reported in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, MACE was reported in 1 patient (0.1 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombocytopenia
In the placebo-controlled trials, for up to 16 weeks, treatment with CIBINQO was associated with a dose-related decrease in platelet count. Maximum effects on platelets were observed within 4 weeks, after which the platelet count returned towards baseline despite continued therapy. In all 5 clinical trials, including the long-term extension trial, 6 subjects (0.9 per 100 patient-years) treated with CIBINQO 200 mg had adverse reactions of thrombocytopenia; no subjects treated with CIBINQO 100 mg had an adverse reaction of thrombocytopenia.
Lymphopenia
In the placebo-controlled trials, for up to 16 weeks, confirmed ALC <500/mm3 occurred in 2 subjects (1.2 per 100 patient-years) treated with CIBINQO 200 mg and 0 subjects treated with CIBINQO 100 mg or placebo. Both cases occurred in the first 4 weeks of exposure.
Lipid Elevations
In the placebo-controlled trials, for up to 16 weeks, there was a dose-related percent increase in low-density lipoprotein cholesterol (LDL-c), total cholesterol, and high-density lipoprotein cholesterol (HDL-c) relative to placebo at Week 4 which remained elevated through the final visit in the treatment period. Adverse reactions related to hyperlipidemia occurred in 1 subject (0.6 per 100 patient-years) exposed to CIBINQO 100 mg, 3 subjects (2.0 per 100 patient-years) exposed to CIBINQO 200 mg.
Retinal Detachment
In the placebo-controlled trials, for up to 16 weeks, retinal detachment occurred in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, retinal detachment occurred in 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 100 mg.
Creatine Phosphokinase Elevations (CPK)
In the placebo-controlled trials, for up to 16 weeks, events of blood CPK increased were reported in 6 subjects (7.5 per 100 patient-years) treated with placebo, 11 subjects (6.9 per 100 patient-years) treated with 100 mg of CIBINQO and 19 subjects (12.3 per 100 patient-years) treated with 200 mg of CIBINQO. Most elevations were transient, there were no reported adverse reactions of rhabdomyolysis.
Pediatric Subjects (12 to less than 18 years of age)
The safety of CIBINQO was assessed in a trial of 284 subjects 12 to less than 18 years of age with moderate-to-severe atopic dermatitis (Trial-AD-4). The safety profile of CIBINQO in these subjects, assessed through the initial treatment period of 12 weeks and the long-term period (213 with at least 52 weeks of abrocitinib exposure), was comparable to the safety profile from trials in adults with atopic dermatitis.
Drug Interactions
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on CIBINQO
Table 4 includes drugs with clinically significant drug interactions affecting CIBINQO.
Strong CYP2C19 Inhibitors | |
Clinical Impact | Coadministration of CIBINQO with strong CYP2C19 inhibitors increases the combined exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO [see Clinical Pharmacology (12.3)]. |
Intervention | Dosage reduction of CIBINQO is recommended when coadministered with strong CYP2C19 inhibitors [see Dosage and Administration (2.5)]. |
Moderate to Strong Inhibitors of both CYP2C19 and CYP2C9 | |
Clinical Impact | Coadministration of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9 increases the exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO [Clinical Pharmacology (12.3)]. |
Intervention | Avoid concomitant use of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9. |
Strong CYP2C19 or CYP2C9 Inducers | |
Clinical Impact | Coadministration of CIBINQO with strong CYP2C19 or CYP2C9 inducers decreases the combined exposure of abrocitinib and its two active metabolites, M1 and M2, which may result in loss of or reduced clinical response [see Clinical Pharmacology (12.3)]. |
Intervention | Avoid concomitant use of CIBINQO with strong CYP2C19 or CYP2C9 inducers. |
7.2 Effects of CIBINQO on Other Drugs
Table 5 includes clinically significant drug interactions affecting other drugs.
P-gp Substrate Where Small Concentration Changes May Lead to Serious or Life-threatening Toxicities | |
Clinical Impact | Coadministration of CIBINQO with P-gp substrate increases plasma concentrations of P-gp substrates and may result in potential adverse reactions of the P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities (e.g., digoxin) [see Clinical Pharmacology (12.3)]. |
Intervention | Monitor appropriately or dose titrate P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities when coadministered with CIBINQO. |
Antiplatelet Therapy Drugs | |
Clinical Impact | Coadministration of CIBINQO with antiplatelet therapy drugs may increase the risk of bleeding with thrombocytopenia [see Warnings and Precautions (5.5) and Clinical Pharmacology (12.2)]. |
Intervention | Antiplatelet drugs, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment are contraindicated with CIBINQO [see Contraindications (4)]. |
Use in Specific Populations
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CIBINQO during pregnancy. Pregnant women exposed to CIBINQO and health care providers are encouraged to call 1-877-311-3770.
Risk Summary
Available data from pregnancies reported in clinical trials with CIBINQO are not sufficient to establish a drug‑associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of abrocitinib to pregnant rats and rabbits during organogenesis at exposure 11 or 4 times the maximum recommended human dose (MRHD) based on AUC comparison, respectively, resulted in maternal dystocia and skeletal variations in rats and no adverse effects in rabbits (see Data).
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risks in the U.S. general population of major birth defects and miscarriages are 2–4% and 15–20% of clinically recognized pregnancies, respectively.
Animal Data
In an embryofetal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, or 60 mg/kg/day during the period of organogenesis. No fetal malformations were observed. Abrocitinib increased the incidence of skeletal variations of short 13th ribs at 30 mg/kg/day (11 times the MRHD based on AUC comparison). Increased embryofetal lethality and additional skeletal variations (cervical arches with reduced ventral processes, thickened ribs, and unossified metatarsals) were noted at 60 mg/kg/day (17 times the MRHD based on AUC comparison).
In an embryofetal development study, abrocitinib was administered orally to pregnant rabbits at doses of 10, 30, or 75 mg/kg/day during the period of organogenesis. No abrocitinib-related maternal or developmental toxicity was noted at doses up to 75 mg/kg/day (4 times the MRHD based on AUC comparison).
In a prenatal and postnatal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, and 60 mg/kg/day beginning on gestation day 6 and continuing through lactation day 20. Dystocia with prolonged parturition and reduced offspring body weights were noted at 30 mg/kg/day (11 times the MRHD based on AUC comparison). Postnatal survival was markedly decreased at 60 mg/kg/day (17 times the MRHD based on AUC comparison). No maternal toxicity was observed at 10 mg/kg/day (2.4 times the MRHD based on AUC comparison). No abrocitinib-related effects on postnatal developmental, neurobehavioral, or reproductive performance of offspring was noted at doses up to 30 mg/kg/day (11 times the MRHD based on AUC comparison).
8.2 Lactation
Risk Summary
There are no data on the presence of abrocitinib in human milk, the effects on the breast-fed infant, or the effects on milk production. Abrocitinib was secreted in milk of lactating rats (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse findings in adults, including risks of serious infections, malignancy, and thrombosis, advise women not to breastfeed during treatment with CIBINQO and for one day after the last dose (approximately 5–6 elimination half-lives).
8.3 Females and Males of Reproductive Potential
Infertility
Females
Based on the findings in rats, oral administration of CIBINQO may impair female fertility. Impaired fertility in female rats was reversible 1 month after cessation of abrocitinib oral administration [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of CIBINQO in pediatric patients 12 years of age and older with atopic dermatitis have been established.
In trials Trial-AD-1 and Trial-AD-2, 124 pediatric subjects 12 to less than 18 years old weighing 25 kg or more with moderate-to-severe atopic dermatitis were enrolled and randomized to receive either CIBINQO 100 mg (N=51), 200 mg (N=48), or matching placebo (N=25) in monotherapy. Additional 284 pediatric subjects 12 to less than 18 years of age weighing 25 kg or more with moderate-to-severe atopic dermatitis, were enrolled and randomized to receive either CIBINQO 100 mg (N=95) or 200 mg (N=94) or matching placebo (N=95) in combination with topical corticosteroids in Trial-AD-4. Efficacy and adverse reaction profile were comparable between the pediatric patients and adults [see Clinical Studies (14) and Adverse Reactions (6.1)].
The safety and effectiveness of CIBINQO have not been established in pediatric patients below 12 years of age.
Juvenile Animal Toxicity Data
In a juvenile animal toxicity study, abrocitinib was administered orally to juvenile rats at doses of 5, 25, and 75 mg/kg/day from postnatal day 10 (approximately equivalent to a human infant) through postnatal day 63 (approximately equivalent to an adolescent). Abrocitinib caused a reversible, dose‑related decrease in the primary spongiosa in the metaphysis of the proximal tibia and distal femur and adverse effects on bone development at all dose levels. Abrocitinib caused irreversible dose-related small or misshapen femoral heads at doses ≥5 mg/kg/day (0.8 times the MRHD based on AUC comparison); irreversibly decreased femur size and caused paw malrotation and limb impairment at doses ≥25 mg/kg/day (7.2 times the MRHD based on AUC comparison); and fractures at 75 mg/kg/day (27 times the MRHD based on AUC comparison).
In a follow-up study, abrocitinib (25 mg/kg/day, at least 4.5 times the MRHD based on AUC comparison) was orally administered to juvenile rats from postnatal day (PND) 10, 15, 21, or 30 through PND day 63. Administration beginning PND 10 caused adverse macroscopic and microscopic bone findings consistent with the previous juvenile animal study. However, administration beginning PND 15 (approximately equivalent to a 6- to 12-month old infant) caused non-adverse reversible microscopic bone findings. No bone findings were noted when administration began on PND 21 or 30 (approximately equivalent to 2- and 6-year old children, respectively).
8.5 Geriatric Use
A total of 145 (4.6%) subjects 65 years of age and older, while 25 (0.8%) were 75 years of age and older, were enrolled in CIBINQO clinical trials. Clinical trials of CIBINQO did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
A higher proportion of subjects 65 years of age and older discontinued from clinical trials compared to younger subjects. Among all subjects exposed to CIBINQO, including the long-term extension trial, confirmed ALC <500/mm3 occurred only in subjects 65 years of age and older. A higher proportion of subjects 65 years of age and older had platelet counts <75,000/mm3. The incidence rate of herpes zoster in subjects 65 years of age and older treated with CIBINQO (7.40 per 100 patient-years) was higher than that of subjects 18 to less than 65 years of age (3.44 per 100 patient-years).
8.6 Renal Impairment
In patients with severe (eGFR <30 mL/min) and moderate (eGFR 30–59 mL/min) renal impairment, the combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2, is increased compared to patients with normal renal function (eGFR ≥90 mL/min) [see Clinical Pharmacology (12.3)]. This may increase the risk of adverse reactions such as infections.
CIBINQO is not recommended for use in patients with severe renal impairment and ESRD including those on renal replacement therapy [see Dosage and Administration (2.3)].
A dosage reduction in patients with moderate renal impairment is recommended. No dosage adjustment is required in patients with mild renal impairment (eGFR 60–89 mL/min) [see Dosage and Administration (2.3)].
CIBINQO has not been studied in subjects on renal replacement therapy. In Phase 3 clinical trials, CIBINQO was not evaluated in subjects with atopic dermatitis with baseline creatinine clearance values less than 40 mL/min.
8.7 Hepatic Impairment
Avoid use of CIBINQO in patients with severe (Child Pugh C) hepatic impairment. In clinical trials, CIBINQO was not evaluated in subjects with severe (Child Pugh C) hepatic impairment.
Dosage adjustment is not required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment based on similar combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2 compared to patients with normal hepatic function [see Clinical Pharmacology (12.3)].
8.8 CYP2C19 Poor Metabolizers
In patients who are CYP2C19 poor metabolizers, the AUC of abrocitinib is increased compared to CYP2C19 normal metabolizers due to reduced metabolic clearance. Dosage reduction of CIBINQO is recommended in patients who are known or suspected to be CYP2C19 poor metabolizers based on genotype or previous history/experience with other CYP2C19 substrates [see Dosage and Administration (2.4) and Clinical Pharmacology (12.5)].
Overdosage
Description
11 DESCRIPTION
CIBINQO (abrocitinib) tablets contain the free base of abrocitinib, a Janus kinase (JAK) inhibitor, for oral administration.
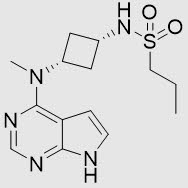
Abrocitinib is a white to pale colored powder with the following chemical name: N-((1s,3s)-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)cyclobutyl)propane-1-sulfonamide
The solubility of abrocitinib in water is 0.04 mg/mL at 25ºC.
Abrocitinib has a molecular weight of 323.42 g/mol and a molecular formula of C14H21N5O2S. The structural formula of abrocitinib is:
Each film-coated tablet contains 50 mg or 100 mg or 200 mg of abrocitinib and the following inactive ingredients: dibasic calcium phosphate anhydrous, hypromellose, iron oxide red, lactose monohydrate, Macrogol, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, titanium dioxide, and triacetin.
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
CIBINQO is a Janus kinase (JAK) inhibitor.
Abrocitinib reversibly inhibits JAK1 by blocking the adenosine triphosphate (ATP) binding site. In a cell-free isolated enzyme assay, abrocitinib was selective for JAK1 over JAK2 (28-fold), JAK3 (>340-fold), and tyrosine kinase (TYK) 2 (43-fold), as well as the broader kinome. The relevance of inhibition of specific JAK enzymes to therapeutic effectiveness is not currently known. Both the parent compound and the active metabolites inhibit JAK1 activity in vitro with similar levels of selectivity.
12.2 Pharmacodynamics
Treatment with CIBINQO was associated with dose-dependent reduction in serum markers of inflammation, including high sensitivity C-reactive protein (hsCRP), interleukin-31 (IL-31) and thymus and activation regulated chemokine (TARC). These changes returned to near baseline within 4 weeks of drug discontinuation.
Effect on Platelet Count
Treatment with CIBINQO was also associated with a transient, dose-dependent decrease in platelet count with the nadir occurring at a median of 24 days after continuous administration of abrocitinib 200 mg once daily. The percent change from baseline of the nadir increases with decreasing baseline platelet counts (-41.2%, -33.4%, and -26.5% for baseline platelet counts of 170, 220, and 270 × 103/mm3, respectively). Partial recovery of platelet count (~40% recovery in platelet count by 12 weeks) occurred without discontinuation of the treatment.
12.3 Pharmacokinetics
Abrocitinib plasma Cmax and AUC increased dose proportionally up to 200 mg. Steady-state plasma concentrations of abrocitinib are achieved within 48 hours after once daily administration.
Absorption
Abrocitinib is absorbed with over 91% extent of oral absorption and absolute oral bioavailability of approximately 60%. The peak plasma concentrations of abrocitinib are reached within 1 hour.
Effect of Food
Coadministration of CIBINQO with a high-fat, high-calorie meal (total 916 calories, with approximate distribution of 55% fat, 29% carbohydrates, and 16% protein) had no clinically relevant effect on abrocitinib exposures (AUC and Cmax of abrocitinib increased by approximately 26% and 29%, respectively, and Tmax was prolonged by 2 hours) [see Dosage and Administration (2.7)].
Distribution
After intravenous administration, the volume of distribution of abrocitinib is approximately 100 L. Approximately 64%, 37% and 29% of circulating abrocitinib and its active metabolites M1 and M2, respectively, are bound to plasma proteins. Abrocitinib and its active metabolites M1 and M2 bind predominantly to albumin and distribute equally between red blood cells and plasma.
Elimination
Abrocitinib is eliminated primarily by metabolic clearance mechanisms. The mean elimination half-lives of abrocitinib and its two active metabolites, M1 and M2, range 3 to 5 hours.
Metabolism
The metabolism of abrocitinib is mediated by multiple CYP enzymes, CYP2C19 (~53%), CYP2C9 (~30%), CYP3A4 (~11%) and CYP2B6 (~6%). In a human radiolabeled study, abrocitinib was the most prevalent circulating species, with two active polar mono-hydroxylated metabolites identified as M1 (3-hydroxypropyl), and M2 (2-hydroxypropyl). Metabolite M1 is less active than abrocitinib while metabolite M2 is as active as the parent. The pharmacologic activity of abrocitinib is attributable to the unbound exposure of parent molecule (~60%) as well as M1 (~10%) and M2 (~30%) in systemic circulation. The sum of unbound exposures of abrocitinib, M1 and M2, each expressed in molar units and adjusted for relative potencies, is referred to as the combined exposure of abrocitinib and its two active metabolites, M1 and M2.
Specific Populations
Body weight, sex, race, and age did not have a clinically meaningful effect on CIBINQO exposure.
Patients with Renal Impairment
In a renal impairment study, subjects with severe (eGFR <30 mL/min as estimated by MDRD equation) and moderate (eGFR 30–59 mL/min, MDRD) renal impairment had approximately 191% and 110% increase in the combined exposure (AUCinf,u) of abrocitinib and its active metabolites, M1 and M2, respectively, compared to subjects with normal renal function (eGFR ≥90 mL/min, MDRD). Based on these results, a clinically significant increase in the combined exposure of abrocitinib and its active metabolites, M1 and M2, is not expected in patients with mild renal impairment (eGFR 60 –89 mL/min, MDRD) [see Dosage and Administration (2.3) and Use in Specific Population (8.6)].
CIBINQO has not been studied in subjects on renal replacement therapy [see Dosage and Administration (2.3) and Use in Specific Population (8.6)]. In Phase 3 clinical trials, CIBINQO was not evaluated in subjects with atopic dermatitis with baseline creatinine clearance values less than 40 mL/min.
Patients with Hepatic Impairment
Subjects with mild hepatic impairment (Child Pugh A) had approximately 4% decrease in the combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2, compared to subjects with normal hepatic function. Subjects with moderate hepatic impairment (Child Pugh B) had approximately 15% increase in the combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2, compared to subjects with normal hepatic function. These changes are not clinically significant. In clinical trials, CIBINQO has not been studied in subjects with severe (Child Pugh C) hepatic impairment, or in subjects screened positive for active hepatitis B or hepatitis C [see Use in Specific Populations (8.7) and Warnings and Precautions (5.1)].
Drug Interaction Studies
Clinical Studies
The effect of coadministered drugs on the pharmacokinetics of abrocitinib is presented in Table 6.
| ||||
Coadministered Drugs | Regimen of Coadministered Drug | Dose of Abrocitinib | Ratio* (90% Confidence Interval) | |
Cmax,u | AUCinf,u | |||
Strong CYP2C19 and moderate CYP3A inhibitor: | 50 mg once daily × 9 days | 100 mg | 1.33 (1.00–1.78) | 1.91 (1.74–2.10) |
Strong CYP2C19, moderate CYP2C9 and CYP3A inhibitor: | 400 mg on Day 1 and 200 mg on Days 2–7 | 100 mg | 1.23 (1.08–1.42) | 2.55† (2.42–2.69) |
Strong CYP Enzymes Inducers: | 600 mg once daily × 8 days | 200 mg | 0.69 (0.50–0.94) | 0.44 (0.41–0.47) |
OAT3 inhibitor: | 1,000 mg twice daily × 3 days | 200 mg | 1.30 (1.04–1.63) | 1.66 (1.52–1.80) |
The effect of abrocitinib on the pharmacokinetics of coadministered drugs is presented in Table 7.
Coadministered Drugs or In Vivo Markers of CYP Activity | Dose Regimen of Abrocitinib | Ratio* (90% Confidence Interval) | |
Cmax | AUCinf | ||
Oral contraceptive: | 200 mg once daily × 9 days | EE: 1.07 (0.99, 1.15) | EE: 1.19 (1.12, 1.26) |
Sensitive CYP3A Substrate: | 200 mg once daily × 7 days | 0.93 (0.84, 1.04) | 0.92 (0.86, 0.99) |
Sensitive P-gp substrate: | 200 mg single dose | 1.40 (0.92, 2.13) | 1.53 (1.09, 2.15) |
Sensitive BCRP and OAT3 substrate: | 200 mg once daily × 3 days | 0.99 (0.86, 1.14) | 1.02 (0.93, 1.12) |
Sensitive MATE1/2K substrate: | 200 mg once daily × 2 days | 0.88 (0.81, 0.96) | 0.93 (0.85, 1.03) |
Coadministration of dabigatran etexilate (a P-gp substrate), with a single dose of CIBINQO 200 mg increased dabigatran AUCinf and Cmax by approximately 53% and 40%, respectively, compared with administration alone. These increases in dabigatran exposure are not considered clinically significant change. However, appropriate dose titration of P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities (e.g., digoxin) when coadministered with the CIBINQO would be needed.
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Abrocitinib and its metabolites M1 and M2 are not inhibitors or inducers of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4.
12.5 Pharmacogenomics
Patients who are CYP2C19 poor metabolizers have little to no CYP2C19 enzyme function compared to CYP2C19 normal metabolizers that have fully functional CYP2C19 enzymes.
After single doses of abrocitinib, CYP2C19 poor metabolizers demonstrated dose-normalized AUC of abrocitinib values that were 2.3-fold higher when compared to CYP2C19 normal metabolizers. Approximately 3–5% of Whites and Blacks and 15 to 20% of Asians are CYP2C19 poor metabolizers [see Dosage and Administration (2.4) and Use in Specific Populations (8.8)].
Nonclinical Toxicology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year oral carcinogenicity study in rats, abrocitinib increased the incidence of benign thymomas in female rats at doses of 10 and 30 mg/kg/day (2.8 and 14 times the MRHD, respectively, based on AUC comparison). Abrocitinib was not carcinogenic in female rats at 3 mg/kg/day (0.6 times the MRHD based on AUC comparison) or male rats at doses up to 30 mg/kg/day (14 times the MRHD based on AUC comparison). Abrocitinib was not carcinogenic in Tg.rasH2 mice at oral doses up to 60 mg/kg/day in males and 75 mg/kg/day in females.
Abrocitinib was not mutagenic in the bacterial mutagenicity assay (Ames assay). Although abrocitinib was aneugenic in the in vitro TK6 micronucleus assay, abrocitinib was not aneugenic or clastogenic in an in vivo rat bone marrow micronucleus assay.
Abrocitinib did not impair male fertility at doses up to 70 mg/kg/day (26 times the MRHD based on AUC comparison) or female fertility at 10 mg/kg/day (2 times the MRHD based on AUC comparison). Abrocitinib impaired female fertility (reducing fertility index, corpora lutea, and implantation sites) at 70 mg/kg/day (29 times the MRHD based on AUC comparison). Impaired fertility in female rats reversed 1 month after cessation of abrocitinib administration.
Clinical Studies
14 CLINICAL STUDIES
The efficacy of CIBINQO as monotherapy and in combination with background topical corticosteroids was evaluated in 4 randomized, double-blind, placebo-controlled trials [Trial-AD-1 (NCT03349060), Trial-AD-2 (NCT03575871), Trial-AD-3 (NCT03720470), and Trial-AD-4 (NCT03796676)] in 1900 subjects (see Table 8). Trial-AD-1 and Trial-AD-2 enrolled adult and pediatric subjects 12 years of age and older. Trial-AD-3 enrolled only adults (≥18 years of age) and Trial-AD-4 enrolled only pediatric subjects 12 to less than 18 years of age. The trials enrolled subjects with moderate-to-severe atopic dermatitis as defined by Investigator’s Global Assessment (IGA) score ≥3, Eczema Area and Severity Index (EASI) score ≥16, body surface area (BSA) involvement ≥10%, and Peak Pruritus Numerical Rating Scale (PP-NRS) ≥4 at the baseline visit prior to randomization.
Baseline Characteristics
In Trial-AD-1, Trial-AD-2, and Trial-AD-3, 53% of subjects were male, 69% of subjects were white, 64% of subjects had a baseline IGA score of 3 (moderate AD), and 36% of subjects had a baseline IGA score of 4 (severe AD). The baseline mean EASI score was 30. The baseline mean age was 36 years old with 8% of subjects 12 to less than 18 years old and 92% of subjects 18 years of age or older. Subjects in these trials were those who had inadequate response to previous topical therapy or were subjects for whom topical treatments were medically inadvisable or who had received systemic therapies including dupilumab. In each of the trials, over 40% of subjects had prior exposure to systemic therapy. In Trial-AD-1 and Trial-AD-2, 6% of the subjects had received dupilumab, whereas prior use of dupilumab was not allowed in Trial-AD-3.
In Trial-AD-4, 49% of subjects were female, 56% of subjects were White, 33% of subjects were Asian and 6% of subjects were Black. The median age was 15 years and the proportion of subjects with severe atopic dermatitis (IGA of 4) was 38%.
Trial Designs and Endpoints
Trial-AD-1, Trial-AD-2, Trial-AD-3, and Trial-AD-4 assessed the co-primary endpoints of IGA and EASI-75 responses at Week 12. The designs of the trials are summarized in Table 8.
| Abbreviations: EASI=Eczema Area and Severity Index; IGA=Investigator’s Global Assessment; QD=once daily; Q2W=once every 2 weeks. | |||
Study Name | Population | Treatment Arms | Co-Primary Endpoints |
Trial-AD-1 12 weeks | Subjects 12 years of age or older (387) | Oral administration of:
| |
Trial-AD-2 12 weeks | Subjects 12 years of age or older (391) | Oral administration of:
| |
Trial-AD-3 16 weeks | Subjects 18 years of age or older (837) | Oral administration of:
Subcutaneous administration of:
All subjects received background topical corticosteroids | |
Trial-AD-4 (combination therapy) 12 weeks | Subjects 12 to less than 18 years of age (285) | Oral administration of:
All subjects received background topical corticosteroids | |
Clinical Response
Monotherapy Trials
The results of the CIBINQO monotherapy trials (Trial-AD-1 and Trial-AD-2) are presented in Table 9.
| Abbreviations: CI=confidence interval; EASI=Eczema Area and Severity Index; IGA=Investigator Global Assessment; QD=once daily. | ||||||
Trial-AD-1 | Trial-AD-2 | |||||
CIBINQO | Placebo | CIBINQO | Placebo | |||
200 mg QD | 100 mg QD | 200 mg QD | 100 mg QD | |||
IGA 0 or 1* | 44% | 24% | 8% | 38% | 28% | 9% |
Difference from Placebo | 36% | 16% | - | 29% | 19% | - |
EASI-75† | 62% | 40% | 12% | 61% | 44% | 10% |
Difference from Placebo | 51% | 28% | - | 50% | 33% | - |
The proportion of subjects achieving PP-NRS4 at Week 2 (defined as an improvement of ≥4 points from baseline in PP-NRS) was higher in subjects treated with CIBINQO monotherapy 200 mg once daily (28% in Trial-AD-1 and 24% in Trial-AD-2) and 100 mg once daily (11% in both trials) compared to placebo (2% in both trials).
A higher proportion of subjects in the CIBINQO monotherapy 100 mg or 200 mg once daily arms compared to placebo achieved improvement in itching at Week 12.
Combination Therapy Trials
The results of CIBINQO in combination with background topical corticosteroids in subjects 18 years of age and older (Trial-AD-3) are presented in Table 10.
| Abbreviations: CI=confidence interval; EASI=Eczema Area and Severity Index; IGA=Investigator Global Assessment; QD=once daily. | |||
% Responders | CIBINQO | Placebo | |
200 mg QD | 100 mg QD | ||
IGA 0 or 1* at Week 12 | 47% | 36% | 14% |
Difference from Placebo | 34% | 23% | - |
EASI-75† at Week 12 | 68% | 58% | 27% |
Difference from Placebo | 41% | 32% | - |
The proportions of subjects achieving PP-NRS4 at Week 2 was higher in subjects treated with CIBINQO 200 mg once daily (30%) and 100 mg once daily (14%) in combination with background medicated topical therapies compared to placebo (8%).
The results of CIBINQO in combination with background topical corticosteroids for pediatric subjects 12 to less than 18 years of age (Trial-AD-4) are presented in Table 11.
| Abbreviations: CI=confidence interval; EASI=Eczema Area and Severity Index; IGA=Investigator Global Assessment; N=number of patients treated; QD=once daily. | |||
% Responders | CIBINQO | Placebo N=95 | |
200 mg QD N=94 | 100 mg QD N=95 | ||
IGA 0 or 1* | 46% | 39% | 24% |
Difference from Placebo (95% CI) | 21% (8%, 34%) | 15% (2%, 28%) | - |
EASI-75† | 71% | 64% | 41% |
Difference from Placebo (95% CI) | 29% (16%, 43%) | 23% (10%, 36%) | - |
The proportion of pediatric subjects 12 to less than 18 years of age achieving PP-NRS4 at Week 2 in Trial-AD-4 was higher with CIBINQO 200 mg once daily (25%) and 100 mg once daily (13%) compared to placebo (8%).
A higher proportion of subjects in the CIBINQO 200 mg once daily arm compared to placebo achieved improvement in itching at Week 12.
Subgroup Analysis (Monotherapy Trials and the Combination Therapy Trial in Subjects 18 Years of Age and Older)
Examination of age, gender, race, weight, and previous systemic AD therapy treatment did not identify differences in response to CIBINQO 100 mg or 200 mg once daily among these subgroups in Trial-AD-1, Trial-AD-2, and Trial-AD-3.
How Supplied/Storage and Handling
16 HOW SUPPLIED/STORAGE AND HANDLING
CIBINQO is supplied as:
Dosage Form | Strength | Description | Bottle Size | NDC Number |
Tablets | 50 mg | Pink, oval tablet debossed with "PFE" on one side and "ABR 50" on the other. | 30 count bottle | 0069-0235-30 |
Tablets | 100 mg | Pink, round tablet debossed with "PFE" on one side and "ABR 100" on the other. | 30 count bottle | 0069-0335-30 |
Tablets | 200 mg | Pink, oval tablet debossed with "PFE" on one side and "ABR 200" on the other. | 30 count bottle | 0069-0435-30 |
Medication Guide
Medication Guide | |||||
What is the most important information I should know about CIBINQO?
You should not start taking CIBINQO if you have any kind of infection unless your healthcare provider tells you it is okay.
| |||||
|
|
| |||
After starting CIBINQO, call your healthcare provider right away if you have any symptoms of an infection. CIBINQO can make you more likely to get infections or make any infections that you have worse. If you get a serious infection, your healthcare provider may stop treatment with CIBINQO until your infection is controlled.
3. Cancer and immune system problems
Tell your healthcare provider if you have ever had any type of cancer.
5. Blood clots
6. Changes in certain laboratory test results
You should not take CIBINQO if your lymphocyte counts, neutrophil counts, red blood cell counts, or platelet counts are too low. Your healthcare provider may stop your CIBINQO treatment for a period of time if needed because of changes in these blood test results. Increased cholesterol levels. You may also have increases in the amount of fat found in your blood. Your healthcare provider should check your cholesterol about 4 weeks after you start CIBINQO, and then as needed. See "What are the possible side effects of CIBINQO?" for more information about side effects. | |||||
What is CIBINQO? It is not known if CIBINQO is safe and effective in children under 12 years of age. | |||||
Do not take CIBINQO if you take medicines that prevent blood clots (antiplatelet medicines), except for low-dose aspirin up to a dose of 81 mg each day during the first 3 months of CIBINQO treatment. | |||||
Before taking CIBINQO, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. CIBINQO and other medicines may affect each other causing side effects. | |||||
How should I take CIBINQO?
| |||||
What are the possible side effects of CIBINQO? See "What is the most important information I should know about CIBINQO?" The most common side effects of CIBINQO include: | |||||
|
| ||||
Separation or tear to the lining of the back part of the eye (retinal detachment) has happened in people with atopic dermatitis treated with CIBINQO. Call your healthcare provider right away if you have any sudden changes in your vision during treatment with CIBINQO.
| |||||
How should I store CIBINQO?
Keep CIBINQO and all medicines out of the reach of children. | |||||
General information about the safe and effective use of CIBINQO. You can ask your pharmacist or healthcare provider for information about CIBINQO that is written for health professionals. | |||||
What are the ingredients in CIBINQO?  LAB-1424-3.0 | |||||
This Medication Guide has been approved by the U.S. Food and Drug Administration. Approved: 12/2023
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Pregnancy Registry
Advise patients to report their pregnancy to 1-877-311-3770 [see Use in Specific Populations (8.1)].
Serious Infections
Inform patients that they may develop infections when taking CIBINQO. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of an infection [see Warnings and Precautions (5.1)].
Advise patients that the risk of herpes zoster is increased in patients treated with CIBINQO and some cases can be serious [see Warnings and Precautions (5.1)].
Malignancies
Inform patients that CIBINQO may increase their risk of certain cancers, including skin cancers. Periodic skin examinations are recommended while using CIBINQO. Advise patients that exposure to sunlight and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen [see Warnings and Precautions (5.3)].
Major Adverse Cardiovascular Events
Inform patients that CIBINQO may increase their risk of major adverse cardiovascular events (MACE) including myocardial infarction, stroke, and cardiovascular death. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events [see Warnings and Precautions (5.4)].
Thrombosis
Advise patients that events of DVT and PE have been reported in clinical trials with CIBINQO. Instruct patients to seek immediate medical attention if they develop any signs or symptoms of a DVT or PE [see Warnings and Precautions (5.5)].
Laboratory Abnormalities
Inform patients that CIBINQO may affect certain lab tests, and that blood tests are required before and during CIBINQO treatment [see Dosage and Administration (2.1) and Warnings and Precautions (5.6)].
Immunizations
Advise patients that vaccination with live vaccines is not recommended during CIBINQO treatment and immediately prior to or after CIBINQO treatment. Instruct patients to inform the healthcare practitioner that they are taking CIBINQO prior to a potential vaccination [see Warnings and Precautions (5.7)].
Retinal Detachment
Inform patients that retinal detachment has been reported in clinical trials for atopic dermatitis in patients who received CIBINQO. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision while receiving CIBINQO [see Adverse Reactions (6.1)].
Infertility
Advise patients who can become pregnant that CIBINQO may impair fertility [see Use in Specific Populations (8.3)].
Lactation
Advise patients not to breastfeed during treatment with CIBINQO [see Use in Specific Populations (8.2)].
Administration
Advise patients not to chew, crush, or split CIBINQO tablets [see Dosage and Administration (2.7)].
Other
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
For Medical Information about CIBINQO, please visit www.pfizermedinfo.com or call 1-800-438-1985.
LAB-1423-3.0
What is the most important information I should know about CIBINQO?
What is the most important information I should know about CIBINQO?
1. Serious infections
CIBINQO is a medicine that affects your immune system. CIBINQO can lower the ability of your immune system to fight infections. Some people have had serious infections while taking CIBINQO or other similar medicines, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have been hospitalized or died from these infections.
- •
- Your healthcare provider should test you for TB before starting treatment with CIBINQO.
- •
- Your healthcare provider should watch you closely for signs and symptoms of TB during treatment with CIBINQO.
You should not start taking CIBINQO if you have any kind of infection unless your healthcare provider tells you it is okay.
You may be at a higher risk of developing shingles (herpes zoster).
Before starting CIBINQO, tell your healthcare provider if you:
- •
- are being treated for an infection
- •
- have had an infection that does not go away or that keeps coming back
- •
- have diabetes, chronic lung disease, HIV, or a weak immune system
- •
- have TB or have been in close contact with someone with TB
- •
- have had shingles (herpes zoster)
- •
- have had hepatitis B or hepatitis C
- •
- live or have lived or have traveled to certain parts of the country (such as the Ohio and Mississippi River valleys and the Southwest) where there is an increased chance for getting certain kinds of fungal infections. These infections may happen or become more severe if you use CIBINQO. Ask your healthcare provider if you do not know if you have lived in an area where these infections are common.
- •
- think you have an infection or have symptoms of an infection such as:
- o
- fever, sweating, or chills
- o
- muscle aches
- o
- cough or shortness of breath
- o
- blood in your phlegm
- o
- weight loss
- o
- warm, red, or painful skin or sores on your body
- o
- diarrhea or stomach pain
- o
- burning when you urinate or urinating more often than usual
- o
- feeling very tired
After starting CIBINQO, call your healthcare provider right away if you have any symptoms of an infection. CIBINQO can make you more likely to get infections or make any infections that you have worse. If you get a serious infection, your healthcare provider may stop treatment with CIBINQO until your infection is controlled.
2. Increased risk of death in people 50 years of age and older who have at least 1 heart disease (cardiovascular) risk factor and are taking a medicine in the class of medicines called Janus kinase (JAK) inhibitors. CIBINQO is a JAK inhibitor medicine.
3. Cancer and immune system problems
CIBINQO may increase your risk of certain cancers by changing the way your immune system works.
- •
- Lymphoma and other cancers, including skin cancers, can happen in people taking CIBINQO.
- •
- People taking a medicine in the class of medicines called Janus kinase (JAK) inhibitors have a higher risk of certain cancers including lymphoma and lung cancer, especially if you are a current or past smoker.
- •
- Follow your healthcare provider's advice about having your skin checked for skin cancer during treatment with CIBINQO. Limit the amount of time you spend in sunlight. Avoid using tanning beds or sunlamps. Wear protective clothing when you are in the sun and use a sunscreen with a high protection factor (SPF 30 and above). This is especially important if your skin is very fair or of you have a family history of skin cancer.
Tell your healthcare provider if you have ever had any type of cancer.
4. Increased risk of major cardiovascular events such as heart attack, stroke or death in people 50 years of age and older who have at least 1 heart disease (cardiovascular) risk factor and taking a medicine in the class of medicines called JAK inhibitors, especially if you are a current or past smoker.
Some people taking CIBINQO have had major cardiovascular events.
Get emergency help right away if you develop any symptoms of a heart attack or stroke during treatment with CIBINQO, including:
- •
- discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back
- •
- severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw
- •
- pain or discomfort in your arms, back, neck, jaw, or stomach
- •
- weakness in one part or on one side of your body
- •
- slurred speech
- •
- shortness of breath with or without chest discomfort
- •
- breaking out in a cold sweat
- •
- nausea or vomiting
- •
- feeling lightheaded
5. Blood clots
Blood clots in the veins of your legs (deep vein thrombosis, DVT) or lungs (pulmonary embolism, PE) can happen in some people taking CIBINQO. This may be life-threatening. Blood clots in the veins of the legs (deep vein thrombosis, DVT) and lungs (pulmonary embolism, PE) have happened more often in people who are 50 years of age and older and with at least 1 heart disease (cardiovascular) risk factor taking a medicine in the class of medicines called Janus kinase (JAK) inhibitors.
- •
- Tell your healthcare provider if you have had blood clots in the veins of your legs or lungs in the past.
- •
- Stop taking CIBINQO and get medical help right away if you have any signs and symptoms of blood clots during treatment with CIBINQO, including:
- o
- swelling, pain or tenderness in one or both legs
- o
- sudden, unexplained chest or upper back pain
- o
- shortness of breath or difficulty breathing
6. Changes in certain laboratory test results
Your healthcare provider should do blood tests before you start taking CIBINQO and during treatment with CIBINQO to check for the following:
- •
- low lymphocyte count. Lymphocytes are white blood cells that help the body fight off infections.
- •
- low neutrophil count. Neutrophils are white blood cells that help the body fight off infections.
- •
- low red blood cell count. This may mean that you have anemia, which may make you feel weak and tired.
- •
- low platelet count. Platelets help form clots and stop or prevent bleeding.
You should not take CIBINQO if your lymphocyte counts, neutrophil counts, red blood cell counts, or platelet counts are too low. Your healthcare provider may stop your CIBINQO treatment for a period of time if needed because of changes in these blood test results.
Increased cholesterol levels. You may also have increases in the amount of fat found in your blood. Your healthcare provider should check your cholesterol about 4 weeks after you start CIBINQO, and then as needed.
See "What are the possible side effects of CIBINQO?" for more information about side effects.
What is CIBINQO?
What is CIBINQO?
It is not known if CIBINQO is safe and effective in children under 12 years of age.
Who should not take CIBINQO?
Who should not take CIBINQO?
Do not take CIBINQO if you take medicines that prevent blood clots (antiplatelet medicines), except for low-dose aspirin up to a dose of 81 mg each day during the first 3 months of CIBINQO treatment.
Before taking CIBINQO, tell your healthcare provider about all of your medical conditions, including if you:
- •
- See "What is the most important information I should know about CIBINQO?"
- •
- have an infection
- •
- are a current or past smoker
- •
- have had a heart attack, other heart problems, or stroke
- •
- have kidney problems or liver problems
- •
- have low platelet counts or white blood cell counts
- •
- have high levels of fat in your blood (high cholesterol)
- •
- have any eye problems, including cataracts or retinal detachment.
- •
- have recently received or are scheduled to receive an immunization (vaccine). People who take CIBINQO should not receive live vaccines.
- •
- are pregnant or plan to become pregnant. It is not known if CIBINQO will harm your unborn baby.
- o
- Pregnancy Exposure Registry. Pfizer has a registry for women who take CIBINQO during pregnancy. The purpose of this registry is to check the health of you and your baby. If you are pregnant or become pregnant during treatment with CIBINQO, talk to your healthcare provider about how you can join this pregnancy registry, or you may contact the registry at 1-877-311-3770 or www.cibinqopregnancyregistry.com.
- •
- are breastfeeding or plan to breastfeed. It is not known if CIBINQO passes into your breast milk. You and your healthcare provider should decide if you will take CIBINQO or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. CIBINQO and other medicines may affect each other causing side effects.
Especially tell your healthcare provider if you take aspirin or any antiplatelet therapies (See the "Who should not take CIBINQO?" section). Ask your healthcare provider if you are unsure.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist whenever you get a new medicine.
How should I take CIBINQO?
How should I take CIBINQO?
- •
- Take CIBINQO exactly as your healthcare provider tells you to take it.
- •
- Take CIBINQO 1 time each day, at about the same time each day.
- •
- Swallow CIBINQO tablets whole with water. Do not split, crush, or chew the tablets.
- •
- You can take CIBINQO with or without food.
- •
- CIBINQO can be used with or without prescribed topical steroid medicines for atopic dermatitis. Prescribed topical medicine are lotions, creams, or ointments applied to your skin.
- •
- If you miss a dose, take the dose as soon as possible. If it is less than 12 hours before the next dose, skip the dose. Take the next dose at your usually scheduled time.
- •
- In case of overdose, get medical help or contact a Poison Center expert right away at 1-800-222-1222. Advice is also available online at poisonhelp.org.
What are the possible side effects of CIBINQO?
What are the possible side effects of CIBINQO?
CIBINQO may cause serious side effects.See "What is the most important information I should know about CIBINQO?"
The most common side effects of CIBINQO include:
- •
- common cold
- •
- nausea
- •
- headache
- •
- herpes simplex including cold sores
- •
- increased blood level of creatine phosphokinase
- •
- dizziness
- •
- urinary tract infection
- •
- tiredness
- •
- acne
- •
- vomiting
- •
- mouth and throat pain
- •
- flu
- •
- stomach flu
- •
- bacterial skin infection (impetigo)
- •
- high blood pressure
- •
- allergic skin rash to something you came into contact with
- •
- stomach-area pain
- •
- shingles
- •
- low platelet count
Separation or tear to the lining of the back part of the eye (retinal detachment) has happened in people with atopic dermatitis treated with CIBINQO. Call your healthcare provider right away if you have any sudden changes in your vision during treatment with CIBINQO.
CIBINQO may cause fertility problems in females, which may affect your ability to get pregnant. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of CIBINQO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Pfizer at 1-800-438-1985.
How should I store CIBINQO?
How should I store CIBINQO?
- •
- Store CIBINQO at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Store CIBINQO in the original package.
- •
- The container has a child resistant closure.
Keep CIBINQO and all medicines out of the reach of children.
General information about the safe and effective use of CIBINQO.
General information about the safe and effective use of CIBINQO.
You can ask your pharmacist or healthcare provider for information about CIBINQO that is written for health professionals.
What are the ingredients in CIBINQO?
What are the ingredients in CIBINQO?
Active ingredient:Inactive ingredients:
LAB-1424-3.0
This Medication Guide has been approved by the U.S. Food and Drug Administration. Approved: 12/2023
Full Patient Information
Full Patient Information
Medication Guide | |||||
What is the most important information I should know about CIBINQO?
You should not start taking CIBINQO if you have any kind of infection unless your healthcare provider tells you it is okay.
| |||||
|
|
| |||
After starting CIBINQO, call your healthcare provider right away if you have any symptoms of an infection. CIBINQO can make you more likely to get infections or make any infections that you have worse. If you get a serious infection, your healthcare provider may stop treatment with CIBINQO until your infection is controlled.
3. Cancer and immune system problems
Tell your healthcare provider if you have ever had any type of cancer.
5. Blood clots
6. Changes in certain laboratory test results
You should not take CIBINQO if your lymphocyte counts, neutrophil counts, red blood cell counts, or platelet counts are too low. Your healthcare provider may stop your CIBINQO treatment for a period of time if needed because of changes in these blood test results. Increased cholesterol levels. You may also have increases in the amount of fat found in your blood. Your healthcare provider should check your cholesterol about 4 weeks after you start CIBINQO, and then as needed. See "What are the possible side effects of CIBINQO?" for more information about side effects. | |||||
What is CIBINQO? It is not known if CIBINQO is safe and effective in children under 12 years of age. | |||||
Do not take CIBINQO if you take medicines that prevent blood clots (antiplatelet medicines), except for low-dose aspirin up to a dose of 81 mg each day during the first 3 months of CIBINQO treatment. | |||||
Before taking CIBINQO, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. CIBINQO and other medicines may affect each other causing side effects. | |||||
How should I take CIBINQO?
| |||||
What are the possible side effects of CIBINQO? See "What is the most important information I should know about CIBINQO?" The most common side effects of CIBINQO include: | |||||
|
| ||||
Separation or tear to the lining of the back part of the eye (retinal detachment) has happened in people with atopic dermatitis treated with CIBINQO. Call your healthcare provider right away if you have any sudden changes in your vision during treatment with CIBINQO.
| |||||
How should I store CIBINQO?
Keep CIBINQO and all medicines out of the reach of children. | |||||
General information about the safe and effective use of CIBINQO. You can ask your pharmacist or healthcare provider for information about CIBINQO that is written for health professionals. | |||||
What are the ingredients in CIBINQO?  LAB-1424-3.0 | |||||
This Medication Guide has been approved by the U.S. Food and Drug Administration. Approved: 12/2023
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Pregnancy Registry
Advise patients to report their pregnancy to 1-877-311-3770 [see Use in Specific Populations (8.1)].
Serious Infections
Inform patients that they may develop infections when taking CIBINQO. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of an infection [see Warnings and Precautions (5.1)].
Advise patients that the risk of herpes zoster is increased in patients treated with CIBINQO and some cases can be serious [see Warnings and Precautions (5.1)].
Malignancies
Inform patients that CIBINQO may increase their risk of certain cancers, including skin cancers. Periodic skin examinations are recommended while using CIBINQO. Advise patients that exposure to sunlight and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen [see Warnings and Precautions (5.3)].
Major Adverse Cardiovascular Events
Inform patients that CIBINQO may increase their risk of major adverse cardiovascular events (MACE) including myocardial infarction, stroke, and cardiovascular death. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events [see Warnings and Precautions (5.4)].
Thrombosis
Advise patients that events of DVT and PE have been reported in clinical trials with CIBINQO. Instruct patients to seek immediate medical attention if they develop any signs or symptoms of a DVT or PE [see Warnings and Precautions (5.5)].
Laboratory Abnormalities
Inform patients that CIBINQO may affect certain lab tests, and that blood tests are required before and during CIBINQO treatment [see Dosage and Administration (2.1) and Warnings and Precautions (5.6)].
Immunizations
Advise patients that vaccination with live vaccines is not recommended during CIBINQO treatment and immediately prior to or after CIBINQO treatment. Instruct patients to inform the healthcare practitioner that they are taking CIBINQO prior to a potential vaccination [see Warnings and Precautions (5.7)].
Retinal Detachment
Inform patients that retinal detachment has been reported in clinical trials for atopic dermatitis in patients who received CIBINQO. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision while receiving CIBINQO [see Adverse Reactions (6.1)].
Infertility
Advise patients who can become pregnant that CIBINQO may impair fertility [see Use in Specific Populations (8.3)].
Lactation
Advise patients not to breastfeed during treatment with CIBINQO [see Use in Specific Populations (8.2)].
Administration
Advise patients not to chew, crush, or split CIBINQO tablets [see Dosage and Administration (2.7)].
Resources
Didn’t find what you were looking for?
Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this product, click the link below to submit your information: Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer product, please call Pfizer Medical Information at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800)-332-1088.