DEPO-SUBQ PROVERA 104® Adverse Reactions
(medroxyprogesterone acetate)
6 ADVERSE REACTIONS
The following important adverse reactions are described in more detail in other sections of the prescribing information:
- •
- Loss of bone mineral density [see Warnings and Precautions (5.1)]
- •
- Arterial and venous thromboembolic disorders [see Warnings and Precautions (5.2)]
- •
- Anaphylaxis [see Warnings and Precautions (5.5)]
- •
- Fluid retention [see Warnings and Precautions (5.6)]
- •
- Delayed return of ovulation or fertility [see Warnings and Precautions (5.8)]
- •
- Depression [see Warnings and Precautions (5.9)]
- •
- Injection site reactions [see Warnings and Precautions (5.10)]
- •
- Bleeding irregularities [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Clinical trials are conducted under widely varying conditions, therefore adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to depo-subQ provera 104 in five clinical trials involving 2325 women including 2043 women who received treatment for contraception (1780 treated up to 1 year and 263 treated for up to 2 years) and 282 women for endometriosis for up to 6 months. In these pooled trials, 9% of women discontinued treatment due to an adverse reaction and the most common reason for discontinuation was dysfunctional uterine bleeding (3%).
Adverse Reactions in the Contraception Adult Studies
Table 1 presents frequently reported adverse reactions (>1%) in the contraception pooled studies. In these studies, the most frequently reported adverse reactions (>5%) were dysfunctional uterine bleeding (e.g., irregular, increased, decreased, or spotting), headache, increased weight, amenorrhea, and injection site reactions (e.g., pain/tenderness, nodule/lump, persistent atrophy/indentation/dimpling or lipodystrophy).
The frequency reported is based on the all-causality incidence in the pooled results of the three contraception studies. Closely related "Adverse Reaction" terms were grouped but individual patients reporting two or more grouped events were only counted once.
| Adverse Reaction | Frequency |
|---|---|
Dysfunctional uterine bleeding (irregular, increase, decrease, spotting) | 18% |
Headache | 9% |
Increased weight (see below) | 7% |
Amenorrhea | 6% |
Injection site reactions (such as pain/tenderness, nodule/lump, persistent atrophy/indentation/dimpling, lipodystrophy, discoloration) | 6% |
Vaginitis, including candidiasis and bacterial | 5% |
Abdominal pain | 4% |
Urinary tract infections | 4% |
Acne | 4% |
Depression | 3% |
Decreased libido | 3% |
Nausea | 3% |
Back pain | 3% |
Breast pain/tenderness | 2% |
Fatigue | 2% |
Anxiety | 1% |
Irritability | 1% |
Dizziness | 1% |
Dysfunctional Uterine Bleeding
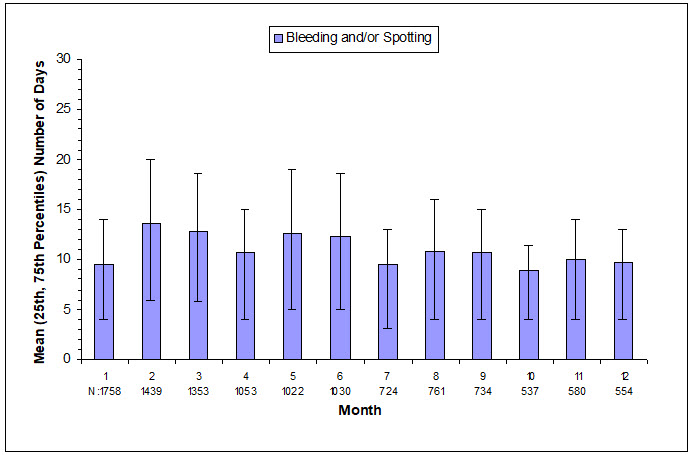
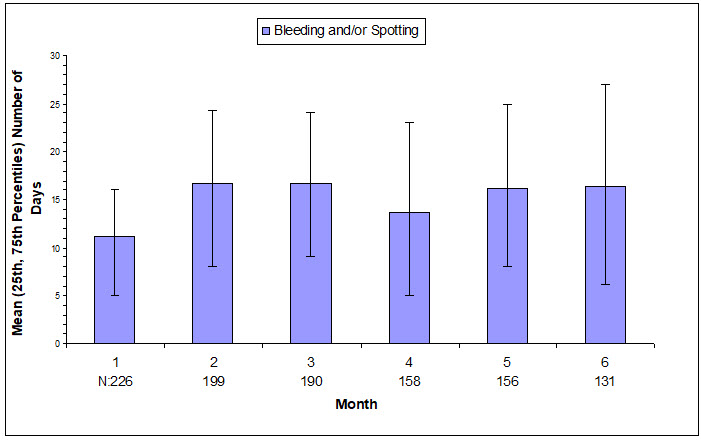
The extent of bleeding and spotting in the three contraception trials is presented in Figure N; data from the endometriosis trials are presented in Figure O [see Warnings and Precautions (5.1)].
Weight Gain
In three large clinical trials, the mean weight gain in depo-subQ provera 104 treated patients was 3.5 lb (1.6 kg) in the first year of use. Half (50%) of women remained within 4.9 lb (2.2 kg) of their initial body weight; 12% of women lost more than 4.9 lb (2.2 kg), and 38% of women gained more than 5.1 lb (2.3 kg). In a small, 2-year study comparing depo-subQ provera 104 to DMPA-IM, the mean weight gain observed for women using depo-subQ provera 104 [7.5 lb (3.4 kg)] was similar to the mean weight gain for women using DMPA-IM [7.7 lb (3.5 kg)].
Other Adverse Reactions Observed in Contraception Clinical Trials with depo-subQ provera 104
Other adverse reactions occurring at an incidence of <1% in women who received depo-subQ provera 104 were as follows:
- •
- Neoplasms benign, malignant and unspecified (including cysts and polyps): breast lump
- •
- Blood and lymphatic system disorders: anemia
- •
- Immune system disorders: drug hypersensitivity
- •
- Metabolism and nutrition disorders: weight decreased, fluid retention
- •
- Nervous system disorders: facial palsy, syncope, paresthesia, somnolence
- •
- Cardiac disorders: tachycardia
- •
- Vascular disorders: hot flushes
- •
- Respiratory, thoracic and mediastinal disorders: asthma, dyspnea
- •
- Gastrointestinal disorders: diarrhea, abdominal distension
- •
- Skin and subcutaneous tissue disorders: urticaria, pruritus, dry skin
- •
- Reproductive system and breast disorders: dysmenorrhea, galactorrhea, dyspareunia
- •
- General disorders and administration site conditions: chest pain
Adverse Reactions in the Endometriosis Adult Studies
The safety profile of depo-subQ provera 104 in endometriosis clinical trials was similar to the safety profile of depo-subQ provera 104 in the contraception studies with the exception of the following adverse reactions which were more frequently reported in patients with endometriosis: abdominal pain, diarrhea, nausea, and back pain.
In endometriosis studies, subjects recorded daily the occurrence and severity of hot flushes. Of the depo-subQ provera 104 users, 29% reported experiencing moderate or severe hot flushes at baseline, 36% at Month 3, and 27% at Month 6. Of the leuprolide users, 33% reported experiencing moderate or severe hot flushes at baseline, 74% at Month 3, and 69% at Month 6.
Adverse Reactions in the Adolescent Contraception Study
Depo-sub-Q provera 104 and DMPA-IM clinical trials reported similar safety profiles in adult study populations (see Table 1 above). Accordingly, a similar safety profile is expected for adolescents receiving depo-subQ provera 104 as for adolescents receiving DMPA-IM.
The safety profile of DMPA-IM for prevention of pregnancy in adolescents was observed to be generally similar to the safety profile of adult women using DMPA-IM for prevention of pregnancy, with the exception of the following adverse reactions which were reported more frequently by adolescents: abdominal pain, diarrhea, back pain, weight increased, depression, headache, and dysmenorrhea.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of DMPA-IM. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- •
- Immune system disorders: anaphylactic reaction, anaphylactoid reaction, angioedema
- •
- Vascular disorders: pulmonary embolism, deep vein thrombosis, thrombophlebitis
- •
- Musculoskeletal and connective tissue disorders: osteoporosis (including osteoporotic fractures)
- •
- Reproductive system and breast disorders: prolonged anovulation, unexpected pregnancy, uterine hyperplasia
- •
- Respiratory, thoracic and mediastinal disorders: hoarseness
- •
- Skin and subcutaneous tissue disorders: increased body odor
- •
- Gastrointestinal disorders: gastrointestinal disturbances
- •
- General disorders and administration site conditions: axillary swelling, chills, thirst
Find DEPO-SUBQ PROVERA 104® medical information:

Find DEPO-SUBQ PROVERA 104® medical information:
DEPO-SUBQ PROVERA 104® Quick Finder
Health Professional Information
Adverse Reactions
6 ADVERSE REACTIONS
The following important adverse reactions are described in more detail in other sections of the prescribing information:
- •
- Loss of bone mineral density [see Warnings and Precautions (5.1)]
- •
- Arterial and venous thromboembolic disorders [see Warnings and Precautions (5.2)]
- •
- Anaphylaxis [see Warnings and Precautions (5.5)]
- •
- Fluid retention [see Warnings and Precautions (5.6)]
- •
- Delayed return of ovulation or fertility [see Warnings and Precautions (5.8)]
- •
- Depression [see Warnings and Precautions (5.9)]
- •
- Injection site reactions [see Warnings and Precautions (5.10)]
- •
- Bleeding irregularities [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Clinical trials are conducted under widely varying conditions, therefore adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to depo-subQ provera 104 in five clinical trials involving 2325 women including 2043 women who received treatment for contraception (1780 treated up to 1 year and 263 treated for up to 2 years) and 282 women for endometriosis for up to 6 months. In these pooled trials, 9% of women discontinued treatment due to an adverse reaction and the most common reason for discontinuation was dysfunctional uterine bleeding (3%).
Adverse Reactions in the Contraception Adult Studies
Table 1 presents frequently reported adverse reactions (>1%) in the contraception pooled studies. In these studies, the most frequently reported adverse reactions (>5%) were dysfunctional uterine bleeding (e.g., irregular, increased, decreased, or spotting), headache, increased weight, amenorrhea, and injection site reactions (e.g., pain/tenderness, nodule/lump, persistent atrophy/indentation/dimpling or lipodystrophy).
The frequency reported is based on the all-causality incidence in the pooled results of the three contraception studies. Closely related "Adverse Reaction" terms were grouped but individual patients reporting two or more grouped events were only counted once.
| Adverse Reaction | Frequency |
|---|---|
Dysfunctional uterine bleeding (irregular, increase, decrease, spotting) | 18% |
Headache | 9% |
Increased weight (see below) | 7% |
Amenorrhea | 6% |
Injection site reactions (such as pain/tenderness, nodule/lump, persistent atrophy/indentation/dimpling, lipodystrophy, discoloration) | 6% |
Vaginitis, including candidiasis and bacterial | 5% |
Abdominal pain | 4% |
Urinary tract infections | 4% |
Acne | 4% |
Depression | 3% |
Decreased libido | 3% |
Nausea | 3% |
Back pain | 3% |
Breast pain/tenderness | 2% |
Fatigue | 2% |
Anxiety | 1% |
Irritability | 1% |
Dizziness | 1% |
Dysfunctional Uterine Bleeding
The extent of bleeding and spotting in the three contraception trials is presented in Figure N; data from the endometriosis trials are presented in Figure O [see Warnings and Precautions (5.1)].
Weight Gain
In three large clinical trials, the mean weight gain in depo-subQ provera 104 treated patients was 3.5 lb (1.6 kg) in the first year of use. Half (50%) of women remained within 4.9 lb (2.2 kg) of their initial body weight; 12% of women lost more than 4.9 lb (2.2 kg), and 38% of women gained more than 5.1 lb (2.3 kg). In a small, 2-year study comparing depo-subQ provera 104 to DMPA-IM, the mean weight gain observed for women using depo-subQ provera 104 [7.5 lb (3.4 kg)] was similar to the mean weight gain for women using DMPA-IM [7.7 lb (3.5 kg)].
Other Adverse Reactions Observed in Contraception Clinical Trials with depo-subQ provera 104
Other adverse reactions occurring at an incidence of <1% in women who received depo-subQ provera 104 were as follows:
- •
- Neoplasms benign, malignant and unspecified (including cysts and polyps): breast lump
- •
- Blood and lymphatic system disorders: anemia
- •
- Immune system disorders: drug hypersensitivity
- •
- Metabolism and nutrition disorders: weight decreased, fluid retention
- •
- Nervous system disorders: facial palsy, syncope, paresthesia, somnolence
- •
- Cardiac disorders: tachycardia
- •
- Vascular disorders: hot flushes
- •
- Respiratory, thoracic and mediastinal disorders: asthma, dyspnea
- •
- Gastrointestinal disorders: diarrhea, abdominal distension
- •
- Skin and subcutaneous tissue disorders: urticaria, pruritus, dry skin
- •
- Reproductive system and breast disorders: dysmenorrhea, galactorrhea, dyspareunia
- •
- General disorders and administration site conditions: chest pain
Adverse Reactions in the Endometriosis Adult Studies
The safety profile of depo-subQ provera 104 in endometriosis clinical trials was similar to the safety profile of depo-subQ provera 104 in the contraception studies with the exception of the following adverse reactions which were more frequently reported in patients with endometriosis: abdominal pain, diarrhea, nausea, and back pain.
In endometriosis studies, subjects recorded daily the occurrence and severity of hot flushes. Of the depo-subQ provera 104 users, 29% reported experiencing moderate or severe hot flushes at baseline, 36% at Month 3, and 27% at Month 6. Of the leuprolide users, 33% reported experiencing moderate or severe hot flushes at baseline, 74% at Month 3, and 69% at Month 6.
Adverse Reactions in the Adolescent Contraception Study
Depo-sub-Q provera 104 and DMPA-IM clinical trials reported similar safety profiles in adult study populations (see Table 1 above). Accordingly, a similar safety profile is expected for adolescents receiving depo-subQ provera 104 as for adolescents receiving DMPA-IM.
The safety profile of DMPA-IM for prevention of pregnancy in adolescents was observed to be generally similar to the safety profile of adult women using DMPA-IM for prevention of pregnancy, with the exception of the following adverse reactions which were reported more frequently by adolescents: abdominal pain, diarrhea, back pain, weight increased, depression, headache, and dysmenorrhea.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of DMPA-IM. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- •
- Immune system disorders: anaphylactic reaction, anaphylactoid reaction, angioedema
- •
- Vascular disorders: pulmonary embolism, deep vein thrombosis, thrombophlebitis
- •
- Musculoskeletal and connective tissue disorders: osteoporosis (including osteoporotic fractures)
- •
- Reproductive system and breast disorders: prolonged anovulation, unexpected pregnancy, uterine hyperplasia
- •
- Respiratory, thoracic and mediastinal disorders: hoarseness
- •
- Skin and subcutaneous tissue disorders: increased body odor
- •
- Gastrointestinal disorders: gastrointestinal disturbances
- •
- General disorders and administration site conditions: axillary swelling, chills, thirst
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.