ZIRABEV Clinical Studies
(bevacizumab-bvzr)
14 CLINICAL STUDIES
14.1 Metastatic Colorectal Cancer
Study AVF2107g
The safety and efficacy of bevacizumab was evaluated in a double-blind, active-controlled study [AVF2107g (NCT00109070)] in 923 patients with previously untreated mCRC who were randomized (1:1:1) to placebo with bolus-IFL (irinotecan 125 mg/m2, fluorouracil 500 mg/m2, and leucovorin 20 mg/m2 given once weekly for 4 weeks every 6 weeks), bevacizumab (5 mg/kg every 2 weeks) with bolus-IFL, or bevacizumab (5 mg/kg every 2 weeks) with fluorouracil and leucovorin. Enrollment to the bevacizumab with fluorouracil and leucovorin arm was discontinued, after enrollment of 110 patients in accordance with the protocol-specified adaptive design. Bevacizumab was continued until disease progression or unacceptable toxicity or for a maximum of 96 weeks. The main outcome measure was overall survival (OS).
The median age was 60 years; 60% were male, 79% were White, 57% had an ECOG performance status of 0, 21% had a rectal primary and 28% received prior adjuvant chemotherapy. The dominant site of disease was extra-abdominal in 56% of patients and was the liver in 38% of patients.
The addition of bevacizumab improved survival across subgroups defined by age (<65 years, ≥65 years) and sex. Results are presented in Table 9 and Figure 1.
| Efficacy Parameter | Bevacizumab with bolus-IFL (N=402) | Placebo with bolus-IFL (N=411) |
|---|---|---|
Overall Survival | ||
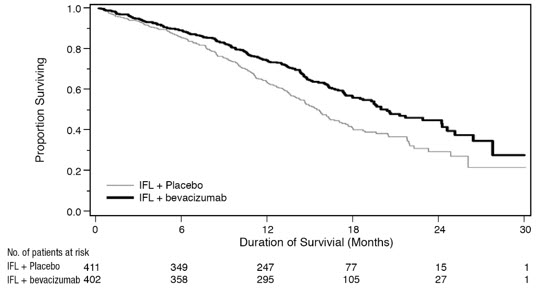
Median, in months | 20.3 | 15.6 |
Hazard ratio (95% CI) | 0.66 (0.54, 0.81) | |
p-value* | < 0.001 | |
Progression-Free Survival | ||
Median, in months | 10.6 | 6.2 |
Hazard ratio (95% CI) | 0.54 (0.45, 0.66) | |
p-value* | < 0.001 | |
Overall Response Rate | ||
Rate (%) | 45% | 35% |
p-value† | < 0.01 | |
Duration of Response | ||
Median, in months | 10.4 | 7.1 |
Figure 1: Kaplan-Meier Curves for Duration of Survival in Metastatic Colorectal Cancer in Study AVF2107g |
 |
Among the 110 patients randomized to bevacizumab with fluorouracil and leucovorin, median OS was 18.3 months, median progression-free survival (PFS) was 8.8 months, overall response rate (ORR) was 39%, and median duration of response was 8.5 months.
Study E3200
The safety and efficacy of bevacizumab were evaluated in a randomized, open-label, active-controlled study [E3200 (NCT00025337)] in 829 patients who were previously treated with irinotecan and fluorouracil for initial therapy for metastatic disease or as adjuvant therapy. Patients were randomized (1:1:1) to FOLFOX4 (Day 1: oxaliplatin 85 mg/m2 and leucovorin 200 mg/m2 concurrently, then fluorouracil 400 mg/m2 bolus followed by 600 mg/m2 continuously; Day 2: leucovorin 200 mg/m2, then fluorouracil 400 mg/m2 bolus followed by 600 mg/m2 continuously; every 2 weeks), bevacizumab (10 mg/kg every 2 weeks prior to FOLFOX4 on Day 1) with FOLFOX4, or bevacizumab alone (10 mg/kg every 2 weeks). Bevacizumab was continued until disease progression or unacceptable toxicity. The main outcome measure was OS.
The bevacizumab alone arm was closed to accrual after enrollment of 244 of the planned 290 patients following a planned interim analysis by the data monitoring committee based on evidence of decreased survival compared to FOLFOX4 alone.
The median age was 61 years; 60% were male, 87% were White, 49% had an ECOG performance status of 0, 26% received prior radiation therapy, and 80% received prior adjuvant chemotherapy, 99% received prior irinotecan with or without fluorouracil for metastatic disease, and 1% received prior irinotecan and fluorouracil as adjuvant therapy.
The addition of bevacizumab to FOLFOX4 resulted in significantly longer survival as compared to FOLFOX4 alone; median OS was 13.0 months vs. 10.8 months [hazard ratio (HR) 0.75 (95% CI: 0.63, 0.89), p-value of 0.001 stratified log-rank test] with clinical benefit seen in subgroups defined by age (<65 years, ≥65 years) and sex. PFS and ORR based on investigator assessment were higher in patients receiving bevacizumab with FOLFOX4.
Study TRC-0301
The activity of bevacizumab with fluorouracil (as bolus or infusion) and leucovorin was evaluated in a single arm study [TRC-0301 (NCT00066846)] enrolling 339 patients with mCRC with disease progression following both irinotecan- and oxaliplatin-based chemotherapy. Seventy-three percent of patients received concurrent bolus fluorouracil and leucovorin. One objective partial response was verified in the first 100 evaluable patients for an ORR of 1% (95% CI: 0%, 5.5%).
Study ML18147
The safety and efficacy of bevacizumab were evaluated in a prospective, randomized, open-label, multinational, controlled study [ML18147 (NCT00700102)] in 820 patients with histologically confirmed mCRC who had progressed on a first-line bevacizumab-containing regimen. Patients were excluded if they progressed within 3 months of initiating first-line chemotherapy and if they received bevacizumab for less than 3 consecutive months in the first-line setting. Patients were randomized (1:1) within 3 months after discontinuing bevacizumab as first-line treatment to receive fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy with or without bevacizumab (5 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks). The choice of second-line treatment was contingent upon first-line chemotherapy. Second-line treatment was administered until progressive disease or unacceptable toxicity. The main outcome measure was OS. A secondary outcome measure was ORR.
The median age was 63 years (21 to 84 years); 64% were male, 52% had an ECOG performance status of 1, 44% had an ECOG performance status of 0, 58% received irinotecan-based therapy as first-line treatment, 55% progressed on first-line treatment within 9 months, and 77% received their last dose of bevacizumab as first-line treatment within 42 days of being randomized. Second-line chemotherapy regimens were generally balanced between each arm.
The addition of bevacizumab to fluoropyrimidine-based chemotherapy resulted in a statistically significant prolongation of OS and PFS. There was no significant difference in ORR. Results are presented in Table 10 and Figure 2.
| Efficacy Parameter | Bevacizumab with Chemotherapy (N=409) | Chemotherapy (N=411) |
|---|---|---|
Overall Survival* | ||
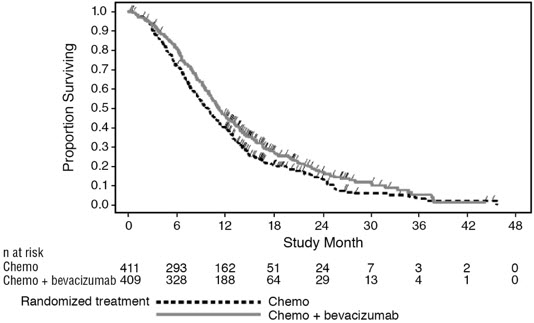
Median, in months | 11.2 | 9.8 |
Hazard ratio (95% CI) | 0.81 (0.69, 0.94) | |
Progression-Free Survival† | ||
Median, in months | 5.7 | 4.0 |
Hazard ratio (95% CI) | 0.68 (0.59, 0.78) | |
Figure 2: Kaplan-Meier Curves for Duration of Survival in Metastatic Colorectal Cancer in Study ML18147 |
 |
14.2 Lack of Efficacy in Adjuvant Treatment of Colon Cancer
Lack of efficacy of bevacizumab as an adjunct to standard chemotherapy for the adjuvant treatment of colon cancer was determined in two randomized, open-label, multicenter clinical studies.
The first study [BO17920 (NCT00112918)] was conducted in 3451 patients with high-risk stage II and III colon cancer, who had undergone surgery for colon cancer with curative intent. Patients were randomized to receive bevacizumab at a dose equivalent to 2.5 mg/kg/week on either a 2-weekly schedule with FOLFOX4 (N=1155) or on a 3-weekly schedule with XELOX (N=1145) or FOLFOX4 alone (N=1151). The main outcome measure was disease free survival (DFS) in patients with stage III colon cancer.
The median age was 58 years; 54% were male, 84% were White and 29% were ≥65 years. Eighty-three percent had stage III disease.
The addition of bevacizumab to chemotherapy did not improve DFS. As compared to FOLFOX4 alone, the proportion of stage III patients with disease recurrence or with death due to disease progression were numerically higher for patients receiving bevacizumab with FOLFOX4 or with XELOX. The hazard ratios for DFS were 1.17 (95% CI: 0.98, 1.39) for bevacizumab with FOLFOX4 versus FOLFOX4 alone and 1.07 (95% CI: 0.90, 1.28) for bevacizumab with XELOX versus FOLFOX4 alone. The hazard ratios for OS were 1.31 (95% CI: 1.03, 1.67) and 1.27 (95% CI: 1, 1.62) for the comparison of bevacizumab with FOLFOX4 versus FOLFOX4 alone and bevacizumab with XELOX versus FOLFOX4 alone, respectively. Similar lack of efficacy for DFS was observed in the bevacizumab-containing arms compared to FOLFOX4 alone in the high-risk stage II cohort.
In a second study [NSABP-C-08 (NCT00096278)], patients with stage II and III colon cancer who had undergone surgery with curative intent, were randomized to receive either bevacizumab administered at a dose equivalent to 2.5 mg/kg/week with mFOLFOX6 (N=1354) or mFOLFOX6 alone (N=1356). The median age was 57 years, 50% were male and 87% White. Seventy-five percent had stage III disease. The main outcome was DFS among stage III patients. The HR for DFS was 0.92 (95% CI: 0.77, 1.10). OS was not significantly improved with the addition of bevacizumab to mFOLFOX6 [HR 0.96 (95% CI: 0.75, 1.22)].
14.3 First-Line Non–Squamous Non–Small Cell Lung Cancer
Study E4599
The safety and efficacy of bevacizumab as first-line treatment of patients with locally advanced, metastatic, or recurrent non-squamous NSCLC was studied in a single, large, randomized, active-controlled, open-label, multicenter study [E4599 (NCT00021060)]. A total of 878 chemotherapy-naïve patients with locally advanced, metastatic or recurrent non–squamous NSCLC were randomized (1:1) to receive six 21-day cycles of paclitaxel (200 mg/m2) and carboplatin (AUC 6) with or without bevacizumab 15 mg/kg. After completing or discontinuing chemotherapy, patients randomized to receive bevacizumab continued to receive bevacizumab alone until disease progression or until unacceptable toxicity. The trial excluded patients with predominant squamous histology (mixed cell type tumors only), CNS metastasis, gross hemoptysis (1/2 teaspoon or more of red blood), unstable angina, or receiving therapeutic anticoagulation. The main outcome measure was duration of survival.
The median age was 63 years; 54% were male, 43% were ≥65 years, and 28% had ≥5% weight loss at study entry. Eleven percent had recurrent disease. Of the 89% with newly diagnosed NSCLC, 12% had Stage IIIB with malignant pleural effusion and 76% had Stage IV disease.
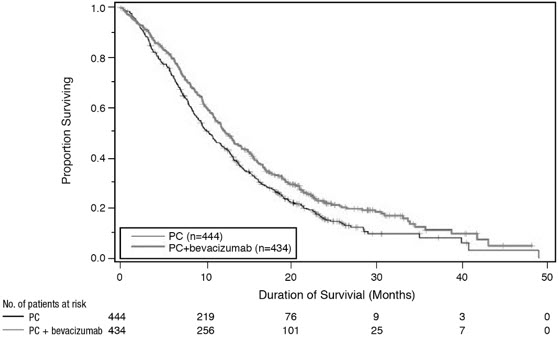
OS was statistically significantly longer for patients receiving bevacizumab with paclitaxel and carboplatin compared with those receiving chemotherapy alone. Median OS was 12.3 months vs. 10.3 months [HR 0.80 (95% CI: 0.68, 0.94), final p-value of 0.013, stratified log-rank test]. Based on investigator assessment which was not independently verified, patients were reported to have longer PFS with bevacizumab with paclitaxel and carboplatin compared to chemotherapy alone. Results are presented in Figure 3.
Figure 3: Kaplan-Meier Curves for Duration of Survival in First-Line Non-Squamous Non-Small Cell Lung Cancer in Study E4599 |
 |
In an exploratory analysis across patient subgroups, the impact of bevacizumab on OS was less robust in the following subgroups: women [HR 0.99 (95% CI: 0.79, 1.25)], patients ≥65 years [HR 0.91 (95% CI: 0.72, 1.14)] and patients with ≥5% weight loss at study entry [HR 0.96 (95% CI: 0.73, 1.26)].
Study BO17704
The safety and efficacy of bevacizumab in patients with locally advanced, metastatic or recurrent non-squamous NSCLC, who had not received prior chemotherapy was studied in another randomized, double-blind, placebo-controlled study [BO17704 (NCT00806923)]. A total of 1043 patients were randomized (1:1:1) to receive cisplatin and gemcitabine with placebo, bevacizumab 7.5 mg/kg or bevacizumab 15 mg/kg. The main outcome measure was PFS. Secondary outcome measure was OS.
The median age was 58 years; 36% were female and 29% were ≥65 years. Eight percent had recurrent disease and 77% had Stage IV disease.
PFS was significantly higher in both bevacizumab-containing arms compared to the placebo arm [HR 0.75 (95% CI: 0.62, 0.91), p-value of 0.0026 for bevacizumab 7.5 mg/kg and HR 0.82 (95% CI: 0.68, 0.98), p-value of 0.0301 for bevacizumab 15 mg/kg]. The addition of bevacizumab to cisplatin and gemcitabine failed to demonstrate an improvement in the duration of OS [HR 0.93 (95% CI: 0.78, 1.11), p-value of 0.420 for bevacizumab 7.5 mg/kg and HR 1.03 (95% CI: 0.86, 1.23), p-value of 0.761 for bevacizumab 15 mg/kg].
14.4 Recurrent Glioblastoma
Study EORTC 26101
The safety and efficacy of bevacizumab were evaluated in a multicenter, randomized (2:1), open-label study in patients with recurrent GBM (EORTC 26101, NCT01290939). Patients with first progression following radiotherapy and temozolomide were randomized (2:1) to receive bevacizumab (10 mg/kg every 2 weeks) with lomustine (90 mg/m2 every 6 weeks) or lomustine (110 mg/m2 every 6 weeks) alone until disease progression or unacceptable toxicity. Randomization was stratified by World Health Organization performance status (0 vs. >0), steroid use (yes vs. no), largest tumor diameter (≤40 vs. >40 mm), and institution. The main outcome measure was OS. Secondary outcome measures were investigator-assessed PFS and ORR per the modified Response Assessment in Neuro-oncology (RANO) criteria, health related quality of life (HRQoL), cognitive function, and corticosteroid use.
A total of 432 patients were randomized to receive lomustine alone (N=149) or bevacizumab with lomustine (N=283). The median age was 57 years; 24.8% of patients were ≥65 years. The majority of patients with were male (61%); 66% had a WHO performance status score >0; and in 56% the largest tumor diameter was ≤40 mm. Approximately 33% of patients randomized to receive lomustine received bevacizumab following documented progression.
No difference in OS (HR 0.91, p-value of 0.4578) was observed between arms; therefore, all secondary outcome measures are descriptive only. PFS was longer in the bevacizumab with lomustine arm [HR 0.52 (95% CI: 0.41, 0.64)] with a median PFS of 4.2 months in the bevacizumab with lomustine arm and 1.5 months in the lomustine arm. Among the 50% of patients receiving corticosteroids at the time of randomization, a higher percentage of patients in the bevacizumab with lomustine arm discontinued corticosteroids (23% vs. 12%).
Study AVF3708g and Study NCI 06-C-0064E
The efficacy and safety of bevacizumab 10 mg/kg every 2 weeks in patients with previously treated GBM were evaluated in one single arm single center study (NCI 06-C-0064E) and a randomized noncomparative multicenter study [AVF3708g(NCT00345163)]. Response rates in both studies were evaluated based on modified WHO criteria that considered corticosteroid use. In AVF3708g, the response rate was 25.9% (95% CI: 17%, 36.1%) with a median duration of response of 4.2 months (95% CI: 3, 5.7). In Study NCI 06-C-0064E, the response rate was 19.6% (95% CI: 10.9%, 31.3%) with a median duration of response of 3.9 months (95% CI: 2.4, 17.4).
14.5 Metastatic Renal Cell Carcinoma
Study BO17705
The safety and efficacy of bevacizumab were evaluated in patients with treatment-naïve mRCC in a multicenter, randomized, double-blind, international study [BO17705 (NCT00738530)] comparing interferon alfa and bevacizumab versus interferon alfa and placebo. A total of 649 patients who had undergone a nephrectomy were randomized (1:1) to receive either bevacizumab (10 mg/kg every 2 weeks; N=327) or placebo (every 2 weeks; N=322) with interferon alfa (9 MIU subcutaneously three times weekly for a maximum of 52 weeks). Patients were treated until disease progression or unacceptable toxicity. The main outcome measure was investigator-assessed PFS. Secondary outcome measures were ORR and OS.
The median age was 60 years (18 to 82 years); 70% were male and 96% were White. The study population was characterized by Motzer scores as follows: 28% favorable (0), 56% intermediate (1–2), 8% poor (3–5), and 7% missing.
PFS was statistically significantly prolonged among patients receiving bevacizumab compared to placebo; median PFS was 10.2 months vs. 5.4 months [HR 0.60 (95% CI: 0.49, 0.72), p-value < 0.0001, stratified log-rank test]. Among the 595 patients with measurable disease, ORR was also significantly higher (30% vs. 12%, p-value < 0.0001, stratified CMH test). There was no improvement in OS based on the final analysis conducted after 444 deaths, with a median OS of 23 months in the patients receiving bevacizumab with interferon alfa and 21 months in patients receiving interferon alone [HR 0.86, (95% CI: 0.72, 1.04)]. Results are presented in Figure 4.
Figure 4: Kaplan-Meier Curves for Progression-Free Survival in Metastatic Renal Cell Carcinoma in Study BO17705 |
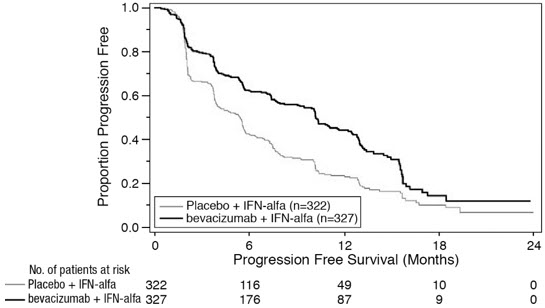 |
14.6 Persistent, Recurrent, or Metastatic Cervical Cancer
Study GOG-0240
The safety and efficacy of bevacizumab were evaluated in patients with persistent, recurrent, or metastatic cervical cancer in a randomized, four-arm, multicenter study comparing bevacizumab with chemotherapy versus chemotherapy alone [GOG-0240 (NCT00803062)]. A total of 452 patients were randomized (1:1:1:1) to receive paclitaxel and cisplatin with or without bevacizumab, or paclitaxel and topotecan with or without bevacizumab.
The dosing regimens for bevacizumab, paclitaxel, cisplatin and topotecan were as follows:
- •
- Day 1: Paclitaxel 135 mg/m2 over 24 hours, Day 2: cisplatin 50 mg/m2 with bevacizumab;
- •
- Day 1: Paclitaxel 175 mg/m2 over 3 hours, Day 2: cisplatin 50 mg/m2 with bevacizumab;
- •
- Day 1: Paclitaxel 175 mg/m2 over 3 hours with cisplatin 50 mg/m2 with bevacizumab;
- •
- Day 1: Paclitaxel 175 mg/m2 over 3 hours with bevacizumab, Days 1–3: topotecan IV 0.75 mg/m2 over 30 minutes.
Patients were treated until disease progression or unacceptable adverse reactions. The main outcome measure was OS. Secondary outcome measures included ORR.
The median age was 48 years (20 to 85 years). Of the 452 patients randomized at baseline, 78% of patients were White, 80% had received prior radiation, 74% had received prior chemotherapy concurrent with radiation, and 32% had a platinum-free interval (PFI) of less than 6 months. Patients had a GOG performance status of 0 (58%) or 1 (42%). Demographic and disease characteristics were balanced across arms.
Results are presented in Figure 5 and Table 11.
Figure 5: Kaplan-Meier Curves for Overall Survival in Persistent, Recurrent, or Metastatic Cervical Cancer in Study GOG-0240 |
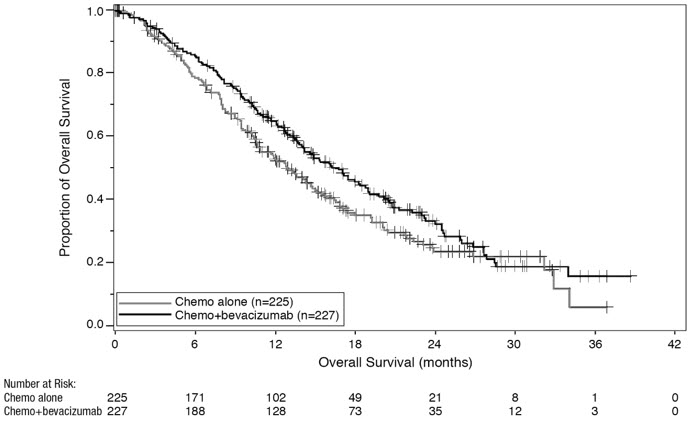 |
| Efficacy Parameter | Bevacizumab with Chemotherapy (N=227) | Chemotherapy (N=225) |
|---|---|---|
Overall Survival | ||
Median, in months* | 16.8 | 12.9 |
Hazard ratio (95% CI) | 0.74 (0.58; 0.94) | |
p-value† | 0.0132 | |
The ORR was higher in patients who received bevacizumab with chemotherapy [45% (95% CI: 39, 52)] compared to patients who received chemotherapy alone [34% (95% CI: 28, 40)].
| Efficacy Parameter | Topotecan and Paclitaxel with or without Bevacizumab (N=223) | Cisplatin and Paclitaxel with or without Bevacizumab (N=229) |
|---|---|---|
| ||
Overall Survival | ||
Median, in months* | 13.3 | 15.5 |
Hazard ratio (95% CI) | 1.15 (0.91, 1.46) | |
p-value | 0.23 | |
The HR for OS with bevacizumab with cisplatin and paclitaxel as compared to cisplatin and paclitaxel alone was 0.72 (95% CI: 0.51, 1.02). The HR for OS with bevacizumab with topotecan and paclitaxel as compared to topotecan and paclitaxel alone was 0.76 (95% CI: 0.55, 1.06).
14.7 Stage III or IV Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer Following Initial Surgical Resection
Study GOG-0218
The safety and efficacy of bevacizumab were evaluated in a multicenter, randomized, double-blind, placebo-controlled, three arm study [Study GOG-0218 (NCT00262847)] evaluating the effect of adding bevacizumab to carboplatin and paclitaxel for the treatment of patients with stage III or IV epithelial ovarian, fallopian tube, or primary peritoneal cancer (N=1873) following initial surgical resection. Patients were randomized (1:1:1) to one of the following arms:
- •
- CPP: carboplatin (AUC 6) and paclitaxel (175 mg/m2) for six cycles, with concurrent placebo started at cycle 2, followed by placebo alone every three weeks for a total of up to 22 cycles of therapy (n=625) or
- •
- CPB15: carboplatin (AUC 6) and paclitaxel (175 mg/m2) for six cycles, with concurrent bevacizumab started at cycle 2, followed by placebo alone every three weeks for a total of up to 22 cycles of therapy (n=625) or
- •
- CPB15+: carboplatin (AUC 6) and paclitaxel (175 mg/m2) for six cycles, with concurrent bevacizumab started at cycle 2, followed by bevacizumab as a single agent every three weeks for a total of up to 22 cycles of therapy (n=623).
The main outcome measure was investigator-assessed PFS. OS was a secondary outcome measure.
The median age was 60 years (range 22–89 years) and 28% of patients were >65 years of age. Overall, approximately 50% of patients had a GOG PS of 0 at baseline, and 43% a GOG PS score of 1. Patients had either epithelial ovarian cancer (83%), primary peritoneal cancer (15%), or fallopian tube cancer (2%). Serous adenocarcinoma was the most common histologic type (85% in CPP and CPB15 arms, 86% in CPB15+ arm). Overall, approximately 34% of patients had resected FIGO Stage III with residual disease <1 cm, 40% had resected Stage III with residual disease >1 cm, and 26% had resected Stage IV disease.
The majority of patients in all three treatment arms received subsequent antineoplastic treatment, 78.1% in the CPP arm, 78.6% in the CPB15 arm, and 73.2% in the CPB15+ arm. A higher proportion of patients in the CPP arm (25.3%) and CPB15 arm (26.6%) received at least one anti-angiogenic (including bevacizumab) treatment after discontinuing from study compared with the CPB15+ arm (15.6%).
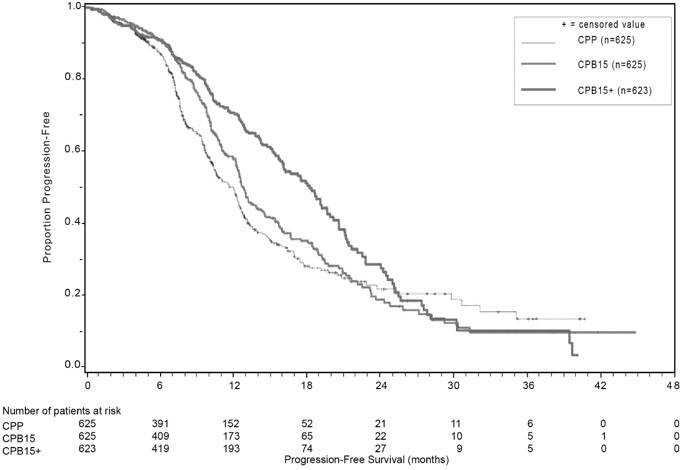
Study results are presented in Table 13 and Figure 6.
| Efficacy Parameter | Bevacizumab with Carboplatin and Paclitaxel followed by Bevacizumab Alone (N=623) | Bevacizumab with Carboplatin and Paclitaxel (N=625) | Carboplatin and Paclitaxel (N=625) |
|---|---|---|---|
| NS=not significant. | |||
Progression-Free Survival per Investigator | |||
Median, in months | 18.2 | 12.8 | 12.0 |
Hazard ratio (95% CI)* | 0.62 (0.52, 0.75) | 0.83 (0.70, 0.98) | |
p-value† | < 0.0001 | NS | |
Overall Survival‡ | |||
Median, in months | 43.8 | 38.8 | 40.6 |
Hazard ratio (95% CI)* | 0.89 (0.76, 1.05) | 1.06 (0.90, 1.24) | |
14.8 Platinum-Resistant Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer
Study MO22224
The safety and efficacy of bevacizumab were evaluated in a multicenter, open-label, randomized study [MO22224 (NCT00976911)] comparing bevacizumab with chemotherapy versus chemotherapy alone in patients with platinum-resistant recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer that recurred within <6 months from the most recent platinum-based therapy (N=361). Patients had received no more than 2 prior chemotherapy regimens. Patients received one of the following chemotherapy regimens at the discretion of the investigator: paclitaxel (80 mg/m2 on days 1, 8, 15 and 22 every 4 weeks); pegylated liposomal doxorubicin (40 mg/m2 on day 1 every 4 weeks); or topotecan (4 mg/m2 on days 1, 8 and 15 every 4 weeks or 1.25 mg/m2 on days 1–5 every 3 weeks). Patients were treated until disease progression, unacceptable toxicity, or withdrawal. Forty percent of patients on the chemotherapy alone arm received bevacizumab alone upon progression. The main outcome measure was investigator-assessed PFS. Secondary outcome measures were ORR and OS.
The median age was 61 years (25 to 84 years) and 37% of patients were ≥65 years. Seventy-nine percent had measurable disease at baseline, 87% had baseline CA-125 levels ≥2 times ULN and 31% had ascites at baseline. Seventy-three percent had a PFI of 3 months to 6 months and 27% had PFI of <3 months. ECOG performance status was 0 for 59%, 1 for 34%, and 2 for 7% of the patients.
The addition of bevacizumab to chemotherapy demonstrated a statistically significant improvement in investigator-assessed PFS, which was supported by a retrospective independent review analysis. Results for the ITT population are presented in Table 14 and Figure 7. Results for the separate chemotherapy cohorts are presented in Table 15.
| Efficacy Parameter | Bevacizumab with Chemotherapy (N=179) | Chemotherapy (N=182) |
|---|---|---|
Progression-Free Survival per Investigator | ||
Median (95% CI), in months | 6.8 (5.6, 7.8) | 3.4 (2.1, 3.8) |
Hazard ratio (95% CI)* | 0.38 (0.30, 0.49) | |
p-value† | < 0.0001 | |
Overall Survival | ||
Median (95% CI), in months | 16.6 (13.7, 19.0) | 13.3 (11.9, 16.4) |
Hazard ratio (95% CI)* | 0.89 (0.69, 1.14) | |
Overall Response Rate | ||
Number of patients with measurable disease at baseline | 142 | 144 |
Rate, % (95% CI) | 28% (21%, 36%) | 13% (7%, 18%) |
Duration of Response | ||
Median, in months | 9.4 | 5.4 |
Figure 7: Kaplan-Meier Curves for Investigator-Assessed Progression-Free Survival in Platinum-Resistant Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Study MO22224 |
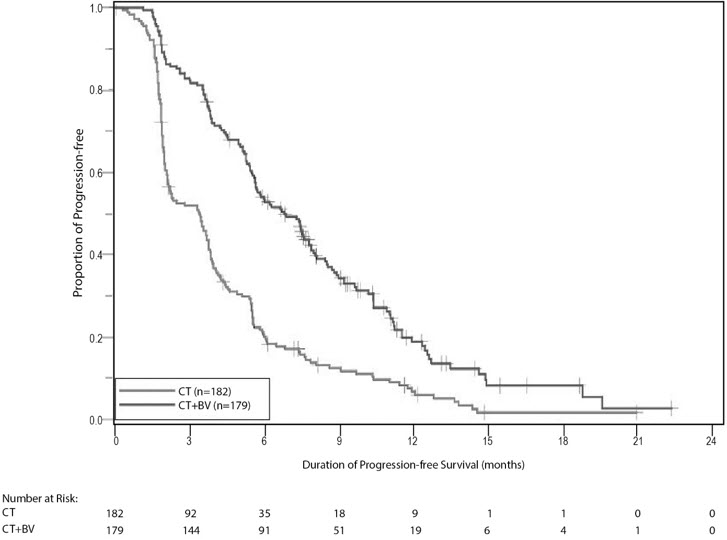 |
| Efficacy Parameter | Paclitaxel | Topotecan | Pegylated Liposomal Doxorubicin | |||
|---|---|---|---|---|---|---|
| Bevacizumab with Chemotherapy | Chemotherapy | Bevacizumab with Chemotherapy | Chemotherapy | Bevacizumab with Chemotherapy | Chemotherapy | |
| (N=60) | (N=55) | (N=57) | (N=63) | (N=62) | (N=64) | |
| NE=Not Estimable. | ||||||
| ||||||
Progression-Free Survival per Investigator | ||||||
Median, in months | 9.6 | 3.9 | 6.2 | 2.1 | 5.1 | 3.5 |
Hazard ratio* | 0.47 | 0.24 | 0.47 | |||
Overall Survival | ||||||
Median, in months | 22.4 | 13.2 | 13.8 | 13.3 | 13.7 | 14.1 |
Hazard ratio* | 0.64 | 1.12 | 0.94 | |||
Overall Response Rate | ||||||
Number of patients with measurable disease at baseline | 45 | 43 | 46 | 50 | 51 | 51 |
Rate, % | 53 | 30 | 17 | 2 | 16 | 8 |
Duration of Response | ||||||
Median, in months | 11.6 | 6.8 | 5.2 | NE | 8.0 | 4.6 |
14.9 Platinum-Sensitive Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer
Study AVF4095g
The safety and efficacy of bevacizumab were evaluated in a randomized, double-blind, placebo-controlled study [AVF4095g (NCT00434642)] studying bevacizumab with chemotherapy versus chemotherapy alone in the treatment of patients with platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who have not received prior chemotherapy in the recurrent setting or prior bevacizumab treatment (N=484). Patients were randomized (1:1) to receive bevacizumab (15 mg/kg day 1) or placebo every 3 weeks with carboplatin (AUC 4, day 1) and gemcitabine (1000 mg/m2 on days 1 and 8) for 6 to 10 cycles followed by bevacizumab or placebo alone until disease progression or unacceptable toxicity. The main outcome measures were investigator-assessed PFS. Secondary outcome measures were ORR and OS.
The median age was 61 years (28 to 87 years) and 37% of patients were ≥65 years. All patients had measurable disease at baseline, 74% had baseline CA-125 levels >ULN (35 U/mL). The PFI was 6 months to 12 months in 42% of patients and >12 months in 58% of patients. The ECOG performance status was 0 or 1 for 99.8% of patients.
A statistically significant prolongation in PFS was demonstrated among patients receiving bevacizumab with chemotherapy compared to those receiving placebo with chemotherapy (Table 16 and Figure 8). Independent radiology review of PFS was consistent with investigator assessment [HR 0.45 (95% CI: 0.35, 0.58)]. OS was not significantly improved with the addition of bevacizumab to chemotherapy [HR 0.95 (95% CI: 0.77, 1.17)].
| Efficacy Parameter | Bevacizumab with Gemcitabine and Carboplatin (N=242) | Placebo with Gemcitabine and Carboplatin (N=242) |
|---|---|---|
Progression-Free Survival | ||
Median, in months | 12.4 | 8.4 |
Hazard ratio | 0.46 | |
p-value | < 0.0001 | |
Overall Response Rate | ||
% patients with overall response | 78% | 57% |
p-value | < 0.0001 | |
Figure 8: Kaplan-Meier Curves for Progression-Free Survival in Platinum-Sensitive Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Study AVF4095g |
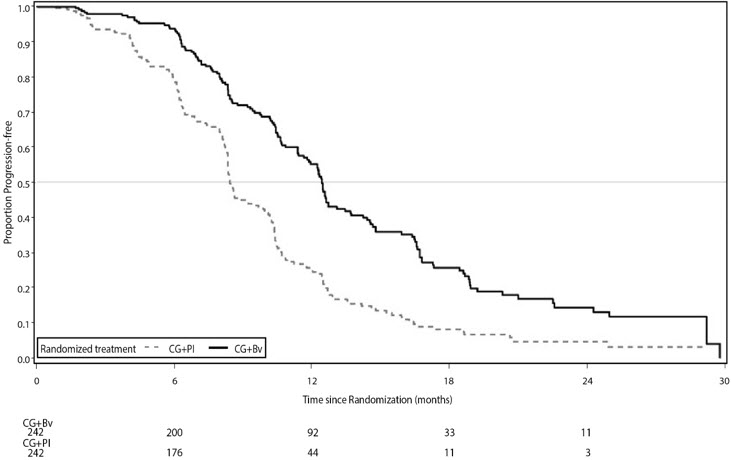 |
Study GOG-0213
The safety and efficacy of bevacizumab were evaluated in a randomized, controlled, open-label study [Study GOG-0213 (NCT00565851)] of bevacizumab with chemotherapy versus chemotherapy alone in the treatment of patients with platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer, who have not received more than one previous regimen of chemotherapy (N=673). Patients were randomized (1:1) to receive carboplatin (AUC 5) and paclitaxel (175 mg/m2 IV over 3 hours) every 3 weeks for 6 to 8 cycles (N=336) or bevacizumab (15 mg/kg) every 3 weeks with carboplatin (AUC 5) and paclitaxel (175 mg/m2 IV over 3 hours) for 6 to 8 cycles followed by bevacizumab (15 mg/kg every 3 weeks) as a single agent until disease progression or unacceptable toxicity. The main outcome measure was OS. Other outcome measures were investigator-assessed PFS, and ORR.
The median age was 60 years (23 to 85 years) and 33% of patients were ≥65 years. Eighty-three percent had measurable disease at baseline and 74% had abnormal CA-125 levels at baseline. Ten percent of patients had received prior bevacizumab. Twenty-six percent had a PFI of 6 months to 12 months and 74% had a PFI of >12 months. GOG performance status was 0 or 1 for 99% of patients.
Results are presented in Table 17 and Figure 9.
| Efficacy Parameter | Bevacizumab with Carboplatin and Paclitaxel (N=337) | Carboplatin and Paclitaxel (N=336) |
|---|---|---|
| ||
Overall Survival | ||
Median, in months | 42.6 | 37.3 |
Hazard ratio (95% CI) | 0.84 (0.69, 1.01) | |
Hazard ratio (95% CI) | 0.82 (0.68, 0.996) | |
Progression-Free Survival | ||
Median, in months | 13.8 | 10.4 |
Hazard ratio (95% CI) | 0.61 (0.51, 0.72) | |
Overall Response Rate | ||
Number of patients with measurable disease at baseline | 274 | 286 |
Rate, % | 213 (78%) | 159 (56%) |
Figure 9: Kaplan-Meier Curves for Overall Survival in Platinum-Sensitive Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Study GOG-0213 |
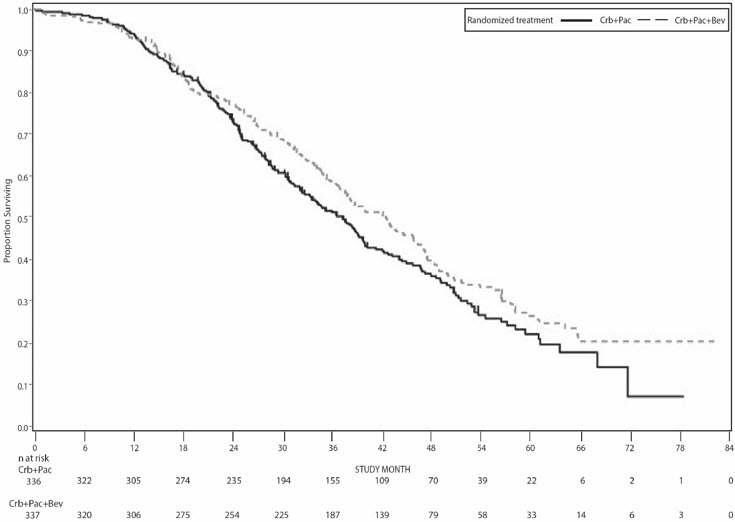 |
Find ZIRABEV medical information:
Find ZIRABEV medical information:
ZIRABEV Quick Finder
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 Metastatic Colorectal Cancer
Study AVF2107g
The safety and efficacy of bevacizumab was evaluated in a double-blind, active-controlled study [AVF2107g (NCT00109070)] in 923 patients with previously untreated mCRC who were randomized (1:1:1) to placebo with bolus-IFL (irinotecan 125 mg/m2, fluorouracil 500 mg/m2, and leucovorin 20 mg/m2 given once weekly for 4 weeks every 6 weeks), bevacizumab (5 mg/kg every 2 weeks) with bolus-IFL, or bevacizumab (5 mg/kg every 2 weeks) with fluorouracil and leucovorin. Enrollment to the bevacizumab with fluorouracil and leucovorin arm was discontinued, after enrollment of 110 patients in accordance with the protocol-specified adaptive design. Bevacizumab was continued until disease progression or unacceptable toxicity or for a maximum of 96 weeks. The main outcome measure was overall survival (OS).
The median age was 60 years; 60% were male, 79% were White, 57% had an ECOG performance status of 0, 21% had a rectal primary and 28% received prior adjuvant chemotherapy. The dominant site of disease was extra-abdominal in 56% of patients and was the liver in 38% of patients.
The addition of bevacizumab improved survival across subgroups defined by age (<65 years, ≥65 years) and sex. Results are presented in Table 9 and Figure 1.
| Efficacy Parameter | Bevacizumab with bolus-IFL (N=402) | Placebo with bolus-IFL (N=411) |
|---|---|---|
Overall Survival | ||
Median, in months | 20.3 | 15.6 |
Hazard ratio (95% CI) | 0.66 (0.54, 0.81) | |
p-value* | < 0.001 | |
Progression-Free Survival | ||
Median, in months | 10.6 | 6.2 |
Hazard ratio (95% CI) | 0.54 (0.45, 0.66) | |
p-value* | < 0.001 | |
Overall Response Rate | ||
Rate (%) | 45% | 35% |
p-value† | < 0.01 | |
Duration of Response | ||
Median, in months | 10.4 | 7.1 |
Figure 1: Kaplan-Meier Curves for Duration of Survival in Metastatic Colorectal Cancer in Study AVF2107g |
 |
Among the 110 patients randomized to bevacizumab with fluorouracil and leucovorin, median OS was 18.3 months, median progression-free survival (PFS) was 8.8 months, overall response rate (ORR) was 39%, and median duration of response was 8.5 months.
Study E3200
The safety and efficacy of bevacizumab were evaluated in a randomized, open-label, active-controlled study [E3200 (NCT00025337)] in 829 patients who were previously treated with irinotecan and fluorouracil for initial therapy for metastatic disease or as adjuvant therapy. Patients were randomized (1:1:1) to FOLFOX4 (Day 1: oxaliplatin 85 mg/m2 and leucovorin 200 mg/m2 concurrently, then fluorouracil 400 mg/m2 bolus followed by 600 mg/m2 continuously; Day 2: leucovorin 200 mg/m2, then fluorouracil 400 mg/m2 bolus followed by 600 mg/m2 continuously; every 2 weeks), bevacizumab (10 mg/kg every 2 weeks prior to FOLFOX4 on Day 1) with FOLFOX4, or bevacizumab alone (10 mg/kg every 2 weeks). Bevacizumab was continued until disease progression or unacceptable toxicity. The main outcome measure was OS.
The bevacizumab alone arm was closed to accrual after enrollment of 244 of the planned 290 patients following a planned interim analysis by the data monitoring committee based on evidence of decreased survival compared to FOLFOX4 alone.
The median age was 61 years; 60% were male, 87% were White, 49% had an ECOG performance status of 0, 26% received prior radiation therapy, and 80% received prior adjuvant chemotherapy, 99% received prior irinotecan with or without fluorouracil for metastatic disease, and 1% received prior irinotecan and fluorouracil as adjuvant therapy.
The addition of bevacizumab to FOLFOX4 resulted in significantly longer survival as compared to FOLFOX4 alone; median OS was 13.0 months vs. 10.8 months [hazard ratio (HR) 0.75 (95% CI: 0.63, 0.89), p-value of 0.001 stratified log-rank test] with clinical benefit seen in subgroups defined by age (<65 years, ≥65 years) and sex. PFS and ORR based on investigator assessment were higher in patients receiving bevacizumab with FOLFOX4.
Study TRC-0301
The activity of bevacizumab with fluorouracil (as bolus or infusion) and leucovorin was evaluated in a single arm study [TRC-0301 (NCT00066846)] enrolling 339 patients with mCRC with disease progression following both irinotecan- and oxaliplatin-based chemotherapy. Seventy-three percent of patients received concurrent bolus fluorouracil and leucovorin. One objective partial response was verified in the first 100 evaluable patients for an ORR of 1% (95% CI: 0%, 5.5%).
Study ML18147
The safety and efficacy of bevacizumab were evaluated in a prospective, randomized, open-label, multinational, controlled study [ML18147 (NCT00700102)] in 820 patients with histologically confirmed mCRC who had progressed on a first-line bevacizumab-containing regimen. Patients were excluded if they progressed within 3 months of initiating first-line chemotherapy and if they received bevacizumab for less than 3 consecutive months in the first-line setting. Patients were randomized (1:1) within 3 months after discontinuing bevacizumab as first-line treatment to receive fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy with or without bevacizumab (5 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks). The choice of second-line treatment was contingent upon first-line chemotherapy. Second-line treatment was administered until progressive disease or unacceptable toxicity. The main outcome measure was OS. A secondary outcome measure was ORR.
The median age was 63 years (21 to 84 years); 64% were male, 52% had an ECOG performance status of 1, 44% had an ECOG performance status of 0, 58% received irinotecan-based therapy as first-line treatment, 55% progressed on first-line treatment within 9 months, and 77% received their last dose of bevacizumab as first-line treatment within 42 days of being randomized. Second-line chemotherapy regimens were generally balanced between each arm.
The addition of bevacizumab to fluoropyrimidine-based chemotherapy resulted in a statistically significant prolongation of OS and PFS. There was no significant difference in ORR. Results are presented in Table 10 and Figure 2.
| Efficacy Parameter | Bevacizumab with Chemotherapy (N=409) | Chemotherapy (N=411) |
|---|---|---|
Overall Survival* | ||
Median, in months | 11.2 | 9.8 |
Hazard ratio (95% CI) | 0.81 (0.69, 0.94) | |
Progression-Free Survival† | ||
Median, in months | 5.7 | 4.0 |
Hazard ratio (95% CI) | 0.68 (0.59, 0.78) | |
Figure 2: Kaplan-Meier Curves for Duration of Survival in Metastatic Colorectal Cancer in Study ML18147 |
 |
14.2 Lack of Efficacy in Adjuvant Treatment of Colon Cancer
Lack of efficacy of bevacizumab as an adjunct to standard chemotherapy for the adjuvant treatment of colon cancer was determined in two randomized, open-label, multicenter clinical studies.
The first study [BO17920 (NCT00112918)] was conducted in 3451 patients with high-risk stage II and III colon cancer, who had undergone surgery for colon cancer with curative intent. Patients were randomized to receive bevacizumab at a dose equivalent to 2.5 mg/kg/week on either a 2-weekly schedule with FOLFOX4 (N=1155) or on a 3-weekly schedule with XELOX (N=1145) or FOLFOX4 alone (N=1151). The main outcome measure was disease free survival (DFS) in patients with stage III colon cancer.
The median age was 58 years; 54% were male, 84% were White and 29% were ≥65 years. Eighty-three percent had stage III disease.
The addition of bevacizumab to chemotherapy did not improve DFS. As compared to FOLFOX4 alone, the proportion of stage III patients with disease recurrence or with death due to disease progression were numerically higher for patients receiving bevacizumab with FOLFOX4 or with XELOX. The hazard ratios for DFS were 1.17 (95% CI: 0.98, 1.39) for bevacizumab with FOLFOX4 versus FOLFOX4 alone and 1.07 (95% CI: 0.90, 1.28) for bevacizumab with XELOX versus FOLFOX4 alone. The hazard ratios for OS were 1.31 (95% CI: 1.03, 1.67) and 1.27 (95% CI: 1, 1.62) for the comparison of bevacizumab with FOLFOX4 versus FOLFOX4 alone and bevacizumab with XELOX versus FOLFOX4 alone, respectively. Similar lack of efficacy for DFS was observed in the bevacizumab-containing arms compared to FOLFOX4 alone in the high-risk stage II cohort.
In a second study [NSABP-C-08 (NCT00096278)], patients with stage II and III colon cancer who had undergone surgery with curative intent, were randomized to receive either bevacizumab administered at a dose equivalent to 2.5 mg/kg/week with mFOLFOX6 (N=1354) or mFOLFOX6 alone (N=1356). The median age was 57 years, 50% were male and 87% White. Seventy-five percent had stage III disease. The main outcome was DFS among stage III patients. The HR for DFS was 0.92 (95% CI: 0.77, 1.10). OS was not significantly improved with the addition of bevacizumab to mFOLFOX6 [HR 0.96 (95% CI: 0.75, 1.22)].
14.3 First-Line Non–Squamous Non–Small Cell Lung Cancer
Study E4599
The safety and efficacy of bevacizumab as first-line treatment of patients with locally advanced, metastatic, or recurrent non-squamous NSCLC was studied in a single, large, randomized, active-controlled, open-label, multicenter study [E4599 (NCT00021060)]. A total of 878 chemotherapy-naïve patients with locally advanced, metastatic or recurrent non–squamous NSCLC were randomized (1:1) to receive six 21-day cycles of paclitaxel (200 mg/m2) and carboplatin (AUC 6) with or without bevacizumab 15 mg/kg. After completing or discontinuing chemotherapy, patients randomized to receive bevacizumab continued to receive bevacizumab alone until disease progression or until unacceptable toxicity. The trial excluded patients with predominant squamous histology (mixed cell type tumors only), CNS metastasis, gross hemoptysis (1/2 teaspoon or more of red blood), unstable angina, or receiving therapeutic anticoagulation. The main outcome measure was duration of survival.
The median age was 63 years; 54% were male, 43% were ≥65 years, and 28% had ≥5% weight loss at study entry. Eleven percent had recurrent disease. Of the 89% with newly diagnosed NSCLC, 12% had Stage IIIB with malignant pleural effusion and 76% had Stage IV disease.
OS was statistically significantly longer for patients receiving bevacizumab with paclitaxel and carboplatin compared with those receiving chemotherapy alone. Median OS was 12.3 months vs. 10.3 months [HR 0.80 (95% CI: 0.68, 0.94), final p-value of 0.013, stratified log-rank test]. Based on investigator assessment which was not independently verified, patients were reported to have longer PFS with bevacizumab with paclitaxel and carboplatin compared to chemotherapy alone. Results are presented in Figure 3.
Figure 3: Kaplan-Meier Curves for Duration of Survival in First-Line Non-Squamous Non-Small Cell Lung Cancer in Study E4599 |
 |
In an exploratory analysis across patient subgroups, the impact of bevacizumab on OS was less robust in the following subgroups: women [HR 0.99 (95% CI: 0.79, 1.25)], patients ≥65 years [HR 0.91 (95% CI: 0.72, 1.14)] and patients with ≥5% weight loss at study entry [HR 0.96 (95% CI: 0.73, 1.26)].
Study BO17704
The safety and efficacy of bevacizumab in patients with locally advanced, metastatic or recurrent non-squamous NSCLC, who had not received prior chemotherapy was studied in another randomized, double-blind, placebo-controlled study [BO17704 (NCT00806923)]. A total of 1043 patients were randomized (1:1:1) to receive cisplatin and gemcitabine with placebo, bevacizumab 7.5 mg/kg or bevacizumab 15 mg/kg. The main outcome measure was PFS. Secondary outcome measure was OS.
The median age was 58 years; 36% were female and 29% were ≥65 years. Eight percent had recurrent disease and 77% had Stage IV disease.
PFS was significantly higher in both bevacizumab-containing arms compared to the placebo arm [HR 0.75 (95% CI: 0.62, 0.91), p-value of 0.0026 for bevacizumab 7.5 mg/kg and HR 0.82 (95% CI: 0.68, 0.98), p-value of 0.0301 for bevacizumab 15 mg/kg]. The addition of bevacizumab to cisplatin and gemcitabine failed to demonstrate an improvement in the duration of OS [HR 0.93 (95% CI: 0.78, 1.11), p-value of 0.420 for bevacizumab 7.5 mg/kg and HR 1.03 (95% CI: 0.86, 1.23), p-value of 0.761 for bevacizumab 15 mg/kg].
14.4 Recurrent Glioblastoma
Study EORTC 26101
The safety and efficacy of bevacizumab were evaluated in a multicenter, randomized (2:1), open-label study in patients with recurrent GBM (EORTC 26101, NCT01290939). Patients with first progression following radiotherapy and temozolomide were randomized (2:1) to receive bevacizumab (10 mg/kg every 2 weeks) with lomustine (90 mg/m2 every 6 weeks) or lomustine (110 mg/m2 every 6 weeks) alone until disease progression or unacceptable toxicity. Randomization was stratified by World Health Organization performance status (0 vs. >0), steroid use (yes vs. no), largest tumor diameter (≤40 vs. >40 mm), and institution. The main outcome measure was OS. Secondary outcome measures were investigator-assessed PFS and ORR per the modified Response Assessment in Neuro-oncology (RANO) criteria, health related quality of life (HRQoL), cognitive function, and corticosteroid use.
A total of 432 patients were randomized to receive lomustine alone (N=149) or bevacizumab with lomustine (N=283). The median age was 57 years; 24.8% of patients were ≥65 years. The majority of patients with were male (61%); 66% had a WHO performance status score >0; and in 56% the largest tumor diameter was ≤40 mm. Approximately 33% of patients randomized to receive lomustine received bevacizumab following documented progression.
No difference in OS (HR 0.91, p-value of 0.4578) was observed between arms; therefore, all secondary outcome measures are descriptive only. PFS was longer in the bevacizumab with lomustine arm [HR 0.52 (95% CI: 0.41, 0.64)] with a median PFS of 4.2 months in the bevacizumab with lomustine arm and 1.5 months in the lomustine arm. Among the 50% of patients receiving corticosteroids at the time of randomization, a higher percentage of patients in the bevacizumab with lomustine arm discontinued corticosteroids (23% vs. 12%).
Study AVF3708g and Study NCI 06-C-0064E
The efficacy and safety of bevacizumab 10 mg/kg every 2 weeks in patients with previously treated GBM were evaluated in one single arm single center study (NCI 06-C-0064E) and a randomized noncomparative multicenter study [AVF3708g(NCT00345163)]. Response rates in both studies were evaluated based on modified WHO criteria that considered corticosteroid use. In AVF3708g, the response rate was 25.9% (95% CI: 17%, 36.1%) with a median duration of response of 4.2 months (95% CI: 3, 5.7). In Study NCI 06-C-0064E, the response rate was 19.6% (95% CI: 10.9%, 31.3%) with a median duration of response of 3.9 months (95% CI: 2.4, 17.4).
14.5 Metastatic Renal Cell Carcinoma
Study BO17705
The safety and efficacy of bevacizumab were evaluated in patients with treatment-naïve mRCC in a multicenter, randomized, double-blind, international study [BO17705 (NCT00738530)] comparing interferon alfa and bevacizumab versus interferon alfa and placebo. A total of 649 patients who had undergone a nephrectomy were randomized (1:1) to receive either bevacizumab (10 mg/kg every 2 weeks; N=327) or placebo (every 2 weeks; N=322) with interferon alfa (9 MIU subcutaneously three times weekly for a maximum of 52 weeks). Patients were treated until disease progression or unacceptable toxicity. The main outcome measure was investigator-assessed PFS. Secondary outcome measures were ORR and OS.
The median age was 60 years (18 to 82 years); 70% were male and 96% were White. The study population was characterized by Motzer scores as follows: 28% favorable (0), 56% intermediate (1–2), 8% poor (3–5), and 7% missing.
PFS was statistically significantly prolonged among patients receiving bevacizumab compared to placebo; median PFS was 10.2 months vs. 5.4 months [HR 0.60 (95% CI: 0.49, 0.72), p-value < 0.0001, stratified log-rank test]. Among the 595 patients with measurable disease, ORR was also significantly higher (30% vs. 12%, p-value < 0.0001, stratified CMH test). There was no improvement in OS based on the final analysis conducted after 444 deaths, with a median OS of 23 months in the patients receiving bevacizumab with interferon alfa and 21 months in patients receiving interferon alone [HR 0.86, (95% CI: 0.72, 1.04)]. Results are presented in Figure 4.
Figure 4: Kaplan-Meier Curves for Progression-Free Survival in Metastatic Renal Cell Carcinoma in Study BO17705 |
 |
14.6 Persistent, Recurrent, or Metastatic Cervical Cancer
Study GOG-0240
The safety and efficacy of bevacizumab were evaluated in patients with persistent, recurrent, or metastatic cervical cancer in a randomized, four-arm, multicenter study comparing bevacizumab with chemotherapy versus chemotherapy alone [GOG-0240 (NCT00803062)]. A total of 452 patients were randomized (1:1:1:1) to receive paclitaxel and cisplatin with or without bevacizumab, or paclitaxel and topotecan with or without bevacizumab.
The dosing regimens for bevacizumab, paclitaxel, cisplatin and topotecan were as follows:
- •
- Day 1: Paclitaxel 135 mg/m2 over 24 hours, Day 2: cisplatin 50 mg/m2 with bevacizumab;
- •
- Day 1: Paclitaxel 175 mg/m2 over 3 hours, Day 2: cisplatin 50 mg/m2 with bevacizumab;
- •
- Day 1: Paclitaxel 175 mg/m2 over 3 hours with cisplatin 50 mg/m2 with bevacizumab;
- •
- Day 1: Paclitaxel 175 mg/m2 over 3 hours with bevacizumab, Days 1–3: topotecan IV 0.75 mg/m2 over 30 minutes.
Patients were treated until disease progression or unacceptable adverse reactions. The main outcome measure was OS. Secondary outcome measures included ORR.
The median age was 48 years (20 to 85 years). Of the 452 patients randomized at baseline, 78% of patients were White, 80% had received prior radiation, 74% had received prior chemotherapy concurrent with radiation, and 32% had a platinum-free interval (PFI) of less than 6 months. Patients had a GOG performance status of 0 (58%) or 1 (42%). Demographic and disease characteristics were balanced across arms.
Results are presented in Figure 5 and Table 11.
Figure 5: Kaplan-Meier Curves for Overall Survival in Persistent, Recurrent, or Metastatic Cervical Cancer in Study GOG-0240 |
 |
| Efficacy Parameter | Bevacizumab with Chemotherapy (N=227) | Chemotherapy (N=225) |
|---|---|---|
Overall Survival | ||
Median, in months* | 16.8 | 12.9 |
Hazard ratio (95% CI) | 0.74 (0.58; 0.94) | |
p-value† | 0.0132 | |
The ORR was higher in patients who received bevacizumab with chemotherapy [45% (95% CI: 39, 52)] compared to patients who received chemotherapy alone [34% (95% CI: 28, 40)].
| Efficacy Parameter | Topotecan and Paclitaxel with or without Bevacizumab (N=223) | Cisplatin and Paclitaxel with or without Bevacizumab (N=229) |
|---|---|---|
| ||
Overall Survival | ||
Median, in months* | 13.3 | 15.5 |
Hazard ratio (95% CI) | 1.15 (0.91, 1.46) | |
p-value | 0.23 | |
The HR for OS with bevacizumab with cisplatin and paclitaxel as compared to cisplatin and paclitaxel alone was 0.72 (95% CI: 0.51, 1.02). The HR for OS with bevacizumab with topotecan and paclitaxel as compared to topotecan and paclitaxel alone was 0.76 (95% CI: 0.55, 1.06).
14.7 Stage III or IV Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer Following Initial Surgical Resection
Study GOG-0218
The safety and efficacy of bevacizumab were evaluated in a multicenter, randomized, double-blind, placebo-controlled, three arm study [Study GOG-0218 (NCT00262847)] evaluating the effect of adding bevacizumab to carboplatin and paclitaxel for the treatment of patients with stage III or IV epithelial ovarian, fallopian tube, or primary peritoneal cancer (N=1873) following initial surgical resection. Patients were randomized (1:1:1) to one of the following arms:
- •
- CPP: carboplatin (AUC 6) and paclitaxel (175 mg/m2) for six cycles, with concurrent placebo started at cycle 2, followed by placebo alone every three weeks for a total of up to 22 cycles of therapy (n=625) or
- •
- CPB15: carboplatin (AUC 6) and paclitaxel (175 mg/m2) for six cycles, with concurrent bevacizumab started at cycle 2, followed by placebo alone every three weeks for a total of up to 22 cycles of therapy (n=625) or
- •
- CPB15+: carboplatin (AUC 6) and paclitaxel (175 mg/m2) for six cycles, with concurrent bevacizumab started at cycle 2, followed by bevacizumab as a single agent every three weeks for a total of up to 22 cycles of therapy (n=623).
The main outcome measure was investigator-assessed PFS. OS was a secondary outcome measure.
The median age was 60 years (range 22–89 years) and 28% of patients were >65 years of age. Overall, approximately 50% of patients had a GOG PS of 0 at baseline, and 43% a GOG PS score of 1. Patients had either epithelial ovarian cancer (83%), primary peritoneal cancer (15%), or fallopian tube cancer (2%). Serous adenocarcinoma was the most common histologic type (85% in CPP and CPB15 arms, 86% in CPB15+ arm). Overall, approximately 34% of patients had resected FIGO Stage III with residual disease <1 cm, 40% had resected Stage III with residual disease >1 cm, and 26% had resected Stage IV disease.
The majority of patients in all three treatment arms received subsequent antineoplastic treatment, 78.1% in the CPP arm, 78.6% in the CPB15 arm, and 73.2% in the CPB15+ arm. A higher proportion of patients in the CPP arm (25.3%) and CPB15 arm (26.6%) received at least one anti-angiogenic (including bevacizumab) treatment after discontinuing from study compared with the CPB15+ arm (15.6%).
Study results are presented in Table 13 and Figure 6.
| Efficacy Parameter | Bevacizumab with Carboplatin and Paclitaxel followed by Bevacizumab Alone (N=623) | Bevacizumab with Carboplatin and Paclitaxel (N=625) | Carboplatin and Paclitaxel (N=625) |
|---|---|---|---|
| NS=not significant. | |||
Progression-Free Survival per Investigator | |||
Median, in months | 18.2 | 12.8 | 12.0 |
Hazard ratio (95% CI)* | 0.62 (0.52, 0.75) | 0.83 (0.70, 0.98) | |
p-value† | < 0.0001 | NS | |
Overall Survival‡ | |||
Median, in months | 43.8 | 38.8 | 40.6 |
Hazard ratio (95% CI)* | 0.89 (0.76, 1.05) | 1.06 (0.90, 1.24) | |
14.8 Platinum-Resistant Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer
Study MO22224
The safety and efficacy of bevacizumab were evaluated in a multicenter, open-label, randomized study [MO22224 (NCT00976911)] comparing bevacizumab with chemotherapy versus chemotherapy alone in patients with platinum-resistant recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer that recurred within <6 months from the most recent platinum-based therapy (N=361). Patients had received no more than 2 prior chemotherapy regimens. Patients received one of the following chemotherapy regimens at the discretion of the investigator: paclitaxel (80 mg/m2 on days 1, 8, 15 and 22 every 4 weeks); pegylated liposomal doxorubicin (40 mg/m2 on day 1 every 4 weeks); or topotecan (4 mg/m2 on days 1, 8 and 15 every 4 weeks or 1.25 mg/m2 on days 1–5 every 3 weeks). Patients were treated until disease progression, unacceptable toxicity, or withdrawal. Forty percent of patients on the chemotherapy alone arm received bevacizumab alone upon progression. The main outcome measure was investigator-assessed PFS. Secondary outcome measures were ORR and OS.
The median age was 61 years (25 to 84 years) and 37% of patients were ≥65 years. Seventy-nine percent had measurable disease at baseline, 87% had baseline CA-125 levels ≥2 times ULN and 31% had ascites at baseline. Seventy-three percent had a PFI of 3 months to 6 months and 27% had PFI of <3 months. ECOG performance status was 0 for 59%, 1 for 34%, and 2 for 7% of the patients.
The addition of bevacizumab to chemotherapy demonstrated a statistically significant improvement in investigator-assessed PFS, which was supported by a retrospective independent review analysis. Results for the ITT population are presented in Table 14 and Figure 7. Results for the separate chemotherapy cohorts are presented in Table 15.
| Efficacy Parameter | Bevacizumab with Chemotherapy (N=179) | Chemotherapy (N=182) |
|---|---|---|
Progression-Free Survival per Investigator | ||
Median (95% CI), in months | 6.8 (5.6, 7.8) | 3.4 (2.1, 3.8) |
Hazard ratio (95% CI)* | 0.38 (0.30, 0.49) | |
p-value† | < 0.0001 | |
Overall Survival | ||
Median (95% CI), in months | 16.6 (13.7, 19.0) | 13.3 (11.9, 16.4) |
Hazard ratio (95% CI)* | 0.89 (0.69, 1.14) | |
Overall Response Rate | ||
Number of patients with measurable disease at baseline | 142 | 144 |
Rate, % (95% CI) | 28% (21%, 36%) | 13% (7%, 18%) |
Duration of Response | ||
Median, in months | 9.4 | 5.4 |
Figure 7: Kaplan-Meier Curves for Investigator-Assessed Progression-Free Survival in Platinum-Resistant Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Study MO22224 |
 |
| Efficacy Parameter | Paclitaxel | Topotecan | Pegylated Liposomal Doxorubicin | |||
|---|---|---|---|---|---|---|
| Bevacizumab with Chemotherapy | Chemotherapy | Bevacizumab with Chemotherapy | Chemotherapy | Bevacizumab with Chemotherapy | Chemotherapy | |
| (N=60) | (N=55) | (N=57) | (N=63) | (N=62) | (N=64) | |
| NE=Not Estimable. | ||||||
| ||||||
Progression-Free Survival per Investigator | ||||||
Median, in months | 9.6 | 3.9 | 6.2 | 2.1 | 5.1 | 3.5 |
Hazard ratio* | 0.47 | 0.24 | 0.47 | |||
Overall Survival | ||||||
Median, in months | 22.4 | 13.2 | 13.8 | 13.3 | 13.7 | 14.1 |
Hazard ratio* | 0.64 | 1.12 | 0.94 | |||
Overall Response Rate | ||||||
Number of patients with measurable disease at baseline | 45 | 43 | 46 | 50 | 51 | 51 |
Rate, % | 53 | 30 | 17 | 2 | 16 | 8 |
Duration of Response | ||||||
Median, in months | 11.6 | 6.8 | 5.2 | NE | 8.0 | 4.6 |
14.9 Platinum-Sensitive Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer
Study AVF4095g
The safety and efficacy of bevacizumab were evaluated in a randomized, double-blind, placebo-controlled study [AVF4095g (NCT00434642)] studying bevacizumab with chemotherapy versus chemotherapy alone in the treatment of patients with platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who have not received prior chemotherapy in the recurrent setting or prior bevacizumab treatment (N=484). Patients were randomized (1:1) to receive bevacizumab (15 mg/kg day 1) or placebo every 3 weeks with carboplatin (AUC 4, day 1) and gemcitabine (1000 mg/m2 on days 1 and 8) for 6 to 10 cycles followed by bevacizumab or placebo alone until disease progression or unacceptable toxicity. The main outcome measures were investigator-assessed PFS. Secondary outcome measures were ORR and OS.
The median age was 61 years (28 to 87 years) and 37% of patients were ≥65 years. All patients had measurable disease at baseline, 74% had baseline CA-125 levels >ULN (35 U/mL). The PFI was 6 months to 12 months in 42% of patients and >12 months in 58% of patients. The ECOG performance status was 0 or 1 for 99.8% of patients.
A statistically significant prolongation in PFS was demonstrated among patients receiving bevacizumab with chemotherapy compared to those receiving placebo with chemotherapy (Table 16 and Figure 8). Independent radiology review of PFS was consistent with investigator assessment [HR 0.45 (95% CI: 0.35, 0.58)]. OS was not significantly improved with the addition of bevacizumab to chemotherapy [HR 0.95 (95% CI: 0.77, 1.17)].
| Efficacy Parameter | Bevacizumab with Gemcitabine and Carboplatin (N=242) | Placebo with Gemcitabine and Carboplatin (N=242) |
|---|---|---|
Progression-Free Survival | ||
Median, in months | 12.4 | 8.4 |
Hazard ratio | 0.46 | |
p-value | < 0.0001 | |
Overall Response Rate | ||
% patients with overall response | 78% | 57% |
p-value | < 0.0001 | |
Figure 8: Kaplan-Meier Curves for Progression-Free Survival in Platinum-Sensitive Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Study AVF4095g |
 |
Study GOG-0213
The safety and efficacy of bevacizumab were evaluated in a randomized, controlled, open-label study [Study GOG-0213 (NCT00565851)] of bevacizumab with chemotherapy versus chemotherapy alone in the treatment of patients with platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer, who have not received more than one previous regimen of chemotherapy (N=673). Patients were randomized (1:1) to receive carboplatin (AUC 5) and paclitaxel (175 mg/m2 IV over 3 hours) every 3 weeks for 6 to 8 cycles (N=336) or bevacizumab (15 mg/kg) every 3 weeks with carboplatin (AUC 5) and paclitaxel (175 mg/m2 IV over 3 hours) for 6 to 8 cycles followed by bevacizumab (15 mg/kg every 3 weeks) as a single agent until disease progression or unacceptable toxicity. The main outcome measure was OS. Other outcome measures were investigator-assessed PFS, and ORR.
The median age was 60 years (23 to 85 years) and 33% of patients were ≥65 years. Eighty-three percent had measurable disease at baseline and 74% had abnormal CA-125 levels at baseline. Ten percent of patients had received prior bevacizumab. Twenty-six percent had a PFI of 6 months to 12 months and 74% had a PFI of >12 months. GOG performance status was 0 or 1 for 99% of patients.
Results are presented in Table 17 and Figure 9.
| Efficacy Parameter | Bevacizumab with Carboplatin and Paclitaxel (N=337) | Carboplatin and Paclitaxel (N=336) |
|---|---|---|
| ||
Overall Survival | ||
Median, in months | 42.6 | 37.3 |
Hazard ratio (95% CI) | 0.84 (0.69, 1.01) | |
Hazard ratio (95% CI) | 0.82 (0.68, 0.996) | |
Progression-Free Survival | ||
Median, in months | 13.8 | 10.4 |
Hazard ratio (95% CI) | 0.61 (0.51, 0.72) | |
Overall Response Rate | ||
Number of patients with measurable disease at baseline | 274 | 286 |
Rate, % | 213 (78%) | 159 (56%) |
Figure 9: Kaplan-Meier Curves for Overall Survival in Platinum-Sensitive Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Study GOG-0213 |
 |
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.