ZAVZPRET™ Clinical Studies
([zavegepant] nasal spray)
14 CLINICAL STUDIES
The efficacy of ZAVZPRET for the acute treatment of migraine with or without aura in adults was demonstrated in two randomized, double-blind, placebo-controlled trials (Study 1 and Study 2). In both studies, patients were instructed to treat a migraine of moderate to severe headache pain intensity. Rescue medication (i.e., NSAIDs, acetaminophen, and/or an antiemetic) was allowed 2 hours after the initial treatment. Other forms of rescue medication such as triptans were not allowed within 48 hours of initial treatment. In Study 1 and Study 2, 13.4% and 13.6% of patients were taking preventive medications for migraine at baseline, respectively. None of the patients were on concomitant preventive medication that act on the CGRP pathway.
In Study 1 (NCT04571060), patients were randomized to receive a single dose of ZAVZPRET 10 mg (N=623) or placebo (N=646). Efficacy was demonstrated with ZAVZPRET 10 mg by an effect on the coprimary endpoints of pain freedom and most bothersome symptom (MBS) freedom at 2 hours after a single dose, compared to placebo. Pain freedom was defined as a reduction of moderate or severe headache pain to no headache pain, and MBS freedom was defined as the absence of the self-identified MBS (i.e., photophobia, phonophobia, or nausea). The most common MBS reported before dosing was photophobia (55%), followed by nausea (28%), and phonophobia (16%).
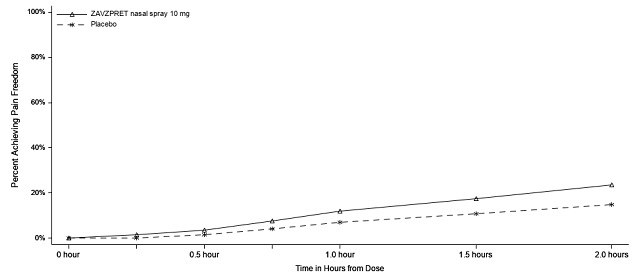
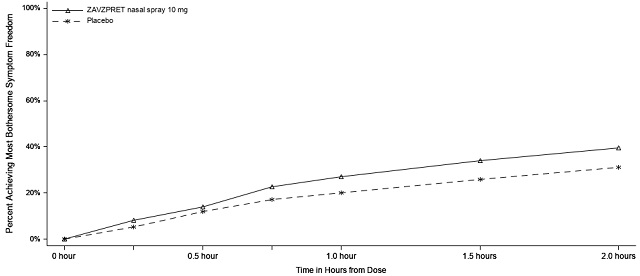
In Study 1, the percentage of patients achieving headache pain freedom and MBS freedom 2 hours after a single dose was statistically significantly greater in patients who received ZAVZPRET compared to those who received placebo (Table 2).
Table 2: Efficacy Endpoints in Study 1
ZAVZPRET 10 mg | Placebo | |
Pain Free at 2 hours | ||
n/N* | 147/623 | 96/646 |
% Responders | 23.6 | 14.9 |
Difference from placebo (%) | 8.8 | |
p-value | <0.001 | |
MBS† Free at 2 hours | ||
n/N* | 247/623 | 201/646 |
% Responders | 39.6 | 31.1 |
Difference from placebo (%) | 8.7 | |
p-value | 0.001 | |
Figures 1 and 2 present the percentage of patients achieving migraine pain freedom and MBS freedom within 2 hours following treatment in Study 1.
Figure 1: Percentage of Patients Achieving Pain Freedom within 2 Hours in Study 1
Figure 2: Percentage of Patients Achieving MBS Freedom within 2 Hours in Study 1
In Study 1, statistically significant effects of ZAVZPRET compared to placebo were demonstrated for the additional efficacy endpoints of pain relief at 2 hours post-dose, return to normal function at 2 hours post-dose, sustained pain freedom from 2 to 48 hours post-dose (Table 3), and phonophobia and photophobia freedom at 2 hours post-dose. Pain relief was defined as a reduction in migraine pain from moderate or severe severity to mild or none. The measurement of the percentage of patients reporting normal function at two hours after dosing was derived from a single item questionnaire, asking patients to select one response on a 4-point scale: normal function, mild impairment, severe impairment, or required bedrest.
Table 3: Additional Efficacy Endpoints in Study 1
ZAVZPRET 10 mg | Placebo | |
Pain Relief at 2 hours | ||
n/N* | 366/623 | 321/646 |
% Responders | 58.7 | 49.7 |
Difference from placebo (%) | 9.0 | |
p-value | 0.001 | |
Percentage of Patients Reporting Normal Function at 2 hours† | ||
n/N* | 204/570 | 152/593 |
% Responders | 35.8 | 25.6 |
Difference from placebo (%) | 10.2 | |
p-value | <0.001 | |
Sustained Pain Freedom from 2 to 48 hours | ||
n/N* | 77/623 | 56/646 |
% Responders | 12.4 | 8.7 |
Difference from placebo (%) | 3.7 | |
p-value | 0.031 | |
The incidence of photophobia and phonophobia was reduced following administration of ZAVZPRET 10 mg as compared to placebo.
In Study 2 (NCT03872453), patients were randomized to receive a single dose of ZAVZPRET 10 mg (n=391) or placebo (n=401).
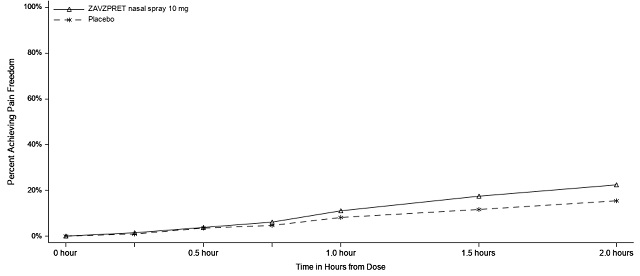
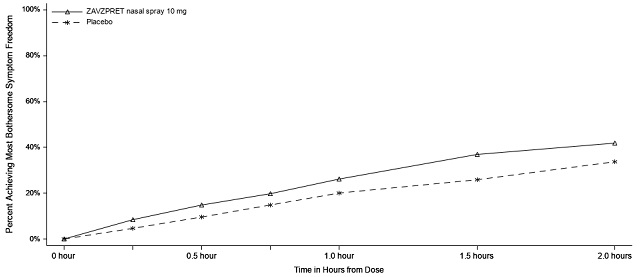
In Study 2, statistically significant efficacy was demonstrated with ZAVZPRET 10 mg by an effect on the coprimary endpoints of pain freedom and most bothersome symptom (MBS) freedom at 2 hours after a single dose, compared to placebo. Pain freedom was observed in 22.5% of patients receiving ZAVZPRET and 15.5% of patients receiving placebo (p-value = 0.011). MBS freedom was observed in 41.9% of patients receiving ZAVZPRET and 33.7% of patients receiving placebo (p-value = 0.016). The most common MBS reported before dosing was photophobia (53%), followed by nausea (31%), and phonophobia (15%).
Table 4: Efficacy Endpoints in Study 2
ZAVZPRET 10 mg | Placebo | |
Pain Free at 2 hours | ||
n/N* | 88/391 | 62/401 |
% Responders | 22.5 | 15.5 |
Difference from placebo (%) | 7.0 | |
p-value | 0.011 | |
MBS† Free at 2 hours | ||
n/N* | 164/391 | 135/401 |
% Responders | 41.9 | 33.7 |
Difference from placebo (%) | 8.3 | |
p-value | 0.016 | |
Figure 3: Percentage of Patients Achieving Pain Freedom within 2 Hours in Study 2
Figure 4: Percentage of Patients Achieving MBS Freedom within 2 Hours in Study 2
Find ZAVZPRET™ medical information:
Find ZAVZPRET™ medical information:
ZAVZPRET™ Quick Finder
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
The efficacy of ZAVZPRET for the acute treatment of migraine with or without aura in adults was demonstrated in two randomized, double-blind, placebo-controlled trials (Study 1 and Study 2). In both studies, patients were instructed to treat a migraine of moderate to severe headache pain intensity. Rescue medication (i.e., NSAIDs, acetaminophen, and/or an antiemetic) was allowed 2 hours after the initial treatment. Other forms of rescue medication such as triptans were not allowed within 48 hours of initial treatment. In Study 1 and Study 2, 13.4% and 13.6% of patients were taking preventive medications for migraine at baseline, respectively. None of the patients were on concomitant preventive medication that act on the CGRP pathway.
In Study 1 (NCT04571060), patients were randomized to receive a single dose of ZAVZPRET 10 mg (N=623) or placebo (N=646). Efficacy was demonstrated with ZAVZPRET 10 mg by an effect on the coprimary endpoints of pain freedom and most bothersome symptom (MBS) freedom at 2 hours after a single dose, compared to placebo. Pain freedom was defined as a reduction of moderate or severe headache pain to no headache pain, and MBS freedom was defined as the absence of the self-identified MBS (i.e., photophobia, phonophobia, or nausea). The most common MBS reported before dosing was photophobia (55%), followed by nausea (28%), and phonophobia (16%).
In Study 1, the percentage of patients achieving headache pain freedom and MBS freedom 2 hours after a single dose was statistically significantly greater in patients who received ZAVZPRET compared to those who received placebo (Table 2).
Table 2: Efficacy Endpoints in Study 1
ZAVZPRET 10 mg | Placebo | |
Pain Free at 2 hours | ||
n/N* | 147/623 | 96/646 |
% Responders | 23.6 | 14.9 |
Difference from placebo (%) | 8.8 | |
p-value | <0.001 | |
MBS† Free at 2 hours | ||
n/N* | 247/623 | 201/646 |
% Responders | 39.6 | 31.1 |
Difference from placebo (%) | 8.7 | |
p-value | 0.001 | |
Figures 1 and 2 present the percentage of patients achieving migraine pain freedom and MBS freedom within 2 hours following treatment in Study 1.
Figure 1: Percentage of Patients Achieving Pain Freedom within 2 Hours in Study 1
Figure 2: Percentage of Patients Achieving MBS Freedom within 2 Hours in Study 1
In Study 1, statistically significant effects of ZAVZPRET compared to placebo were demonstrated for the additional efficacy endpoints of pain relief at 2 hours post-dose, return to normal function at 2 hours post-dose, sustained pain freedom from 2 to 48 hours post-dose (Table 3), and phonophobia and photophobia freedom at 2 hours post-dose. Pain relief was defined as a reduction in migraine pain from moderate or severe severity to mild or none. The measurement of the percentage of patients reporting normal function at two hours after dosing was derived from a single item questionnaire, asking patients to select one response on a 4-point scale: normal function, mild impairment, severe impairment, or required bedrest.
Table 3: Additional Efficacy Endpoints in Study 1
ZAVZPRET 10 mg | Placebo | |
Pain Relief at 2 hours | ||
n/N* | 366/623 | 321/646 |
% Responders | 58.7 | 49.7 |
Difference from placebo (%) | 9.0 | |
p-value | 0.001 | |
Percentage of Patients Reporting Normal Function at 2 hours† | ||
n/N* | 204/570 | 152/593 |
% Responders | 35.8 | 25.6 |
Difference from placebo (%) | 10.2 | |
p-value | <0.001 | |
Sustained Pain Freedom from 2 to 48 hours | ||
n/N* | 77/623 | 56/646 |
% Responders | 12.4 | 8.7 |
Difference from placebo (%) | 3.7 | |
p-value | 0.031 | |
The incidence of photophobia and phonophobia was reduced following administration of ZAVZPRET 10 mg as compared to placebo.
In Study 2 (NCT03872453), patients were randomized to receive a single dose of ZAVZPRET 10 mg (n=391) or placebo (n=401).
In Study 2, statistically significant efficacy was demonstrated with ZAVZPRET 10 mg by an effect on the coprimary endpoints of pain freedom and most bothersome symptom (MBS) freedom at 2 hours after a single dose, compared to placebo. Pain freedom was observed in 22.5% of patients receiving ZAVZPRET and 15.5% of patients receiving placebo (p-value = 0.011). MBS freedom was observed in 41.9% of patients receiving ZAVZPRET and 33.7% of patients receiving placebo (p-value = 0.016). The most common MBS reported before dosing was photophobia (53%), followed by nausea (31%), and phonophobia (15%).
Table 4: Efficacy Endpoints in Study 2
ZAVZPRET 10 mg | Placebo | |
Pain Free at 2 hours | ||
n/N* | 88/391 | 62/401 |
% Responders | 22.5 | 15.5 |
Difference from placebo (%) | 7.0 | |
p-value | 0.011 | |
MBS† Free at 2 hours | ||
n/N* | 164/391 | 135/401 |
% Responders | 41.9 | 33.7 |
Difference from placebo (%) | 8.3 | |
p-value | 0.016 | |
Figure 3: Percentage of Patients Achieving Pain Freedom within 2 Hours in Study 2
Figure 4: Percentage of Patients Achieving MBS Freedom within 2 Hours in Study 2
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.