VFEND® Description
(voriconazole)
11 DESCRIPTION
VFEND (voriconazole), an azole antifungal agent is available as a lyophilized powder for solution for intravenous infusion, film-coated tablets for oral administration, and as a powder for oral suspension. The structural formula is:
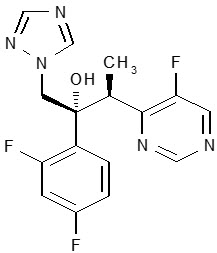
Voriconazole is designated chemically as (2R,3S)-2-(2, 4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol with an empirical formula of C16H14F3N5O and a molecular weight of 349.3.
Voriconazole drug substance is a white to light-colored powder.
VFEND I.V. is a white lyophilized powder containing nominally 200 mg voriconazole and 3200 mg sulfobutyl ether beta-cyclodextrin sodium in a 30 mL Type I clear glass vial.
VFEND I.V. is intended for administration by intravenous infusion. It is a single-dose, unpreserved product. Vials containing 200 mg lyophilized voriconazole are intended for reconstitution with Water for Injection to produce a solution containing 10 mg/mL VFEND and 160 mg/mL of sulfobutyl ether beta-cyclodextrin sodium. The resultant solution is further diluted prior to administration as an intravenous infusion [see Dosage and Administration (2)].
VFEND Tablets contain 50 mg or 200 mg of voriconazole. The inactive ingredients include croscarmellose sodium, lactose monohydrate, magnesium stearate, povidone, pregelatinized starch, and a coating containing hypromellose, lactose monohydrate, titanium dioxide, and triacetin.
VFEND for Oral Suspension is a white to off-white powder providing a white to off-white orange-flavored suspension when reconstituted. Bottles containing 45 grams powder for oral suspension, which contain 3 g of voriconazole, are intended for reconstitution with water to produce a suspension containing 40 mg/mL voriconazole. The inactive ingredients include anhydrous citric acid, colloidal silicon dioxide, natural orange flavor, sodium benzoate, sodium citrate dihydrate, sucrose, titanium dioxide, and xanthan gum.
Find VFEND® medical information:
Find VFEND® medical information:
VFEND® Quick Finder
Health Professional Information
Description
11 DESCRIPTION
VFEND (voriconazole), an azole antifungal agent is available as a lyophilized powder for solution for intravenous infusion, film-coated tablets for oral administration, and as a powder for oral suspension. The structural formula is:

Voriconazole is designated chemically as (2R,3S)-2-(2, 4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol with an empirical formula of C16H14F3N5O and a molecular weight of 349.3.
Voriconazole drug substance is a white to light-colored powder.
VFEND I.V. is a white lyophilized powder containing nominally 200 mg voriconazole and 3200 mg sulfobutyl ether beta-cyclodextrin sodium in a 30 mL Type I clear glass vial.
VFEND I.V. is intended for administration by intravenous infusion. It is a single-dose, unpreserved product. Vials containing 200 mg lyophilized voriconazole are intended for reconstitution with Water for Injection to produce a solution containing 10 mg/mL VFEND and 160 mg/mL of sulfobutyl ether beta-cyclodextrin sodium. The resultant solution is further diluted prior to administration as an intravenous infusion [see Dosage and Administration (2)].
VFEND Tablets contain 50 mg or 200 mg of voriconazole. The inactive ingredients include croscarmellose sodium, lactose monohydrate, magnesium stearate, povidone, pregelatinized starch, and a coating containing hypromellose, lactose monohydrate, titanium dioxide, and triacetin.
VFEND for Oral Suspension is a white to off-white powder providing a white to off-white orange-flavored suspension when reconstituted. Bottles containing 45 grams powder for oral suspension, which contain 3 g of voriconazole, are intended for reconstitution with water to produce a suspension containing 40 mg/mL voriconazole. The inactive ingredients include anhydrous citric acid, colloidal silicon dioxide, natural orange flavor, sodium benzoate, sodium citrate dihydrate, sucrose, titanium dioxide, and xanthan gum.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.