tobramycin injection, USP Dosage and Administration
DOSAGE AND ADMINISTRATION
Tobramycin Injection, USP may be given intramuscularly or intravenously. Recommended dosages are the same for both routes. The patient's pretreatment body weight should be obtained for calculation of correct dosage. It is desirable to measure both peak and trough serum concentrations (see WARNINGS box and PRECAUTIONS).
Administration for Patients with Normal Renal Function
Adults with Serious Infections: 3 mg/kg/day in 3 equal doses every 8 hours (see Table 1).
Adults with Life-Threatening Infections: Up to 5 mg/kg/day may be administered in 3 or 4 equal doses (see Table 1). The dosage should be reduced to 3 mg/kg/day as soon as clinically indicated. To prevent increased toxicity due to excessive blood levels, dosage should not exceed 5 mg/kg/day unless serum levels are monitored (see WARNINGS box and PRECAUTIONS).
For | Usual Dose for | Maximum Dose for Life- | |||
1 mg/kg q8h | 1.66 mg/kg q8h | ||||
mg/dose | mL/dose* | mg/dose | mL/dose* | ||
kg | lb | q8h | q8h | ||
120 | 264 | 120 mg | 3 mL | 200 mg | 5 mL |
115 | 253 | 115 mg | 2.9 mL | 191 mg | 4.75 mL |
110 | 242 | 110 mg | 2.75 mL | 183 mg | 4.5 mL |
105 | 231 | 105 mg | 2.6 mL | 175 mg | 4.4 mL |
100 | 220 | 100 mg | 2.5 mL | 166 mg | 4.2 mL |
95 | 209 | 95 mg | 2.4 mL | 158 mg | 4 mL |
90 | 198 | 90 mg | 2.25 mL | 150 mg | 3.75 mL |
85 | 187 | 85 mg | 2.1 mL | 141 mg | 3.5 mL |
80 | 176 | 80 mg | 2 mL | 133 mg | 3.3 mL |
75 | 165 | 75 mg | 1.9 mL | 125 mg | 3.1 mL |
70 | 154 | 70 mg | 1.75 mL | 116 mg | 2.9 mL |
65 | 143 | 65 mg | 1.6 mL | 108 mg | 2.7 mL |
60 | 132 | 60 mg | 1.5 mL | 100 mg | 2.5 mL |
55 | 121 | 55 mg | 1.4 mL | 91 mg | 2.25 mL |
50 | 110 | 50 mg | 1.25 mL | 83 mg | 2.1 mL |
45 | 99 | 45 mg | 1.1 mL | 75 mg | 1.9 mL |
40 | 88 | 40 mg | 1 mL | 66 mg | 1.6 mL |
* Applicable to all product forms except Tobramycin Injection, USP, 10 mg/mL (Pediatric). | |||||
Pediatric Patients (Greater Than 1 Week of Age):
6 to 7.5 mg/kg/day in 3 or 4 equally divided doses (2 to 2.5 mg/kg every 8 hours or 1.5 to 1.89 mg/kg every 6 hours).
Premature or Full-Term Neonates 1 Week of Age or Less: Up to 4 mg/kg/day may be administered in 2 equal doses every 12 hours.
It is desirable to limit treatment to a short term. The usual duration of treatment is 7 to 10 days. A longer course of therapy may be necessary in difficult and complicated infections. In such cases, monitoring of renal, auditory, and vestibular functions is advised, because neurotoxicity is more likely to occur when treatment is extended longer than 10 days.
Dosage in Patients with Cystic Fibrosis
In patients with cystic fibrosis, altered pharmacokinetics may result in reduced serum concentration of aminoglycosides. Measurement of tobramycin serum concentration during treatment is especially important as a basis for determining appropriate dose. In patients with severe cystic fibrosis, an initial dosing regimen of 10 mg/kg/day in 4 equally divided doses is recommended. This dosing regimen is suggested only as a guide. The serum levels of tobramycin should be measured directly during treatment due to a wide interpatient variability.
Administration for Patients with Impaired Renal Function
Whenever possible, serum tobramycin concentrations should be monitored during therapy.
Following a loading dose of 1 mg/kg, subsequent dosage in these patients must be adjusted, either with reduced doses administered at 8-hour intervals or with normal doses given at prolonged intervals. Both of these methods are suggested as guides to be used when serum levels of tobramycin cannot be measured directly. They are based on either the creatinine clearance or the serum creatinine of the patient, because these values correlate with the half-life of tobramycin. The dosage schedules derived from either method should be used in conjunction with careful clinical and laboratory observations of the patient and should be modified as necessary. Neither method should be used when dialysis is being performed.
Reduced Dosage at 8-hour Intervals
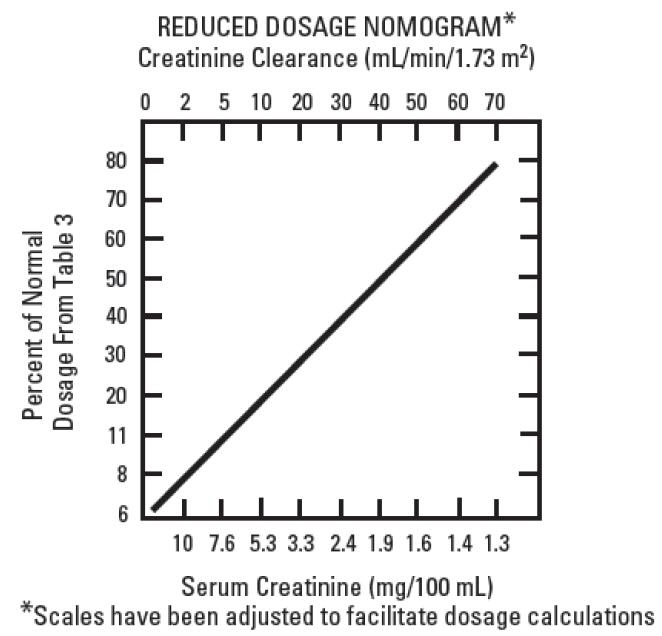
When the creatinine clearance rate is 70 mL or less per minute or when the serum creatinine value is known, the amount of the reduced dose can be determined by multiplying the normal dose from Table 1 by the percent of normal dose from the accompanying nomogram.
An alternate rough guide for determining reduced dosage at 8-hour intervals (for patients whose steady-state serum creatinine values are known) is to divide the normally recommended dose by the patient's serum creatinine.
Normal Dosage at Prolonged Intervals
If the creatinine clearance rate is not available and the patient's condition is stable, a dosage frequency in hours for the dosage given in Table 1 can be determined by multiplying the patient's serum creatinine by 6.
Dosage in Obese Patients
The appropriate dose may be calculated by using the patient's estimated lean body weight plus 40% of the excess as the basic weight on which to figure mg/kg.
Intramuscular Administration
Tobramycin Injection, USP may be administered by withdrawing the appropriate dose directly from a vial. Tobramycin Sulfate in 0.9% Sodium Chloride is not intended for intramuscular administration.
Intravenous Administration
For intravenous administration, the usual volume of diluent (0.9% Sodium Chloride Injection or 5% Dextrose Injection) is 50 to 100 mL for adult doses. For pediatric patients, the volume of diluent should be proportionately less than for adults. The diluted solution usually should be infused over a period of 20 to 60 minutes. Infusion periods of less than 20 minutes are not recommended because peak serum levels may exceed 12 mcg/mL (see WARNINGS box).
Tobramycin Injection, USP should not be physically premixed with other drugs but should be administered separately according to the recommended dose and route.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Find tobramycin injection, USP medical information:
Find tobramycin injection, USP medical information:
tobramycin injection, USP Quick Finder
Health Professional Information
Dosage and Administration
DOSAGE AND ADMINISTRATION
Tobramycin Injection, USP may be given intramuscularly or intravenously. Recommended dosages are the same for both routes. The patient's pretreatment body weight should be obtained for calculation of correct dosage. It is desirable to measure both peak and trough serum concentrations (see WARNINGS box and PRECAUTIONS).
Administration for Patients with Normal Renal Function
Adults with Serious Infections: 3 mg/kg/day in 3 equal doses every 8 hours (see Table 1).
Adults with Life-Threatening Infections: Up to 5 mg/kg/day may be administered in 3 or 4 equal doses (see Table 1). The dosage should be reduced to 3 mg/kg/day as soon as clinically indicated. To prevent increased toxicity due to excessive blood levels, dosage should not exceed 5 mg/kg/day unless serum levels are monitored (see WARNINGS box and PRECAUTIONS).
For | Usual Dose for | Maximum Dose for Life- | |||
1 mg/kg q8h | 1.66 mg/kg q8h | ||||
mg/dose | mL/dose* | mg/dose | mL/dose* | ||
kg | lb | q8h | q8h | ||
120 | 264 | 120 mg | 3 mL | 200 mg | 5 mL |
115 | 253 | 115 mg | 2.9 mL | 191 mg | 4.75 mL |
110 | 242 | 110 mg | 2.75 mL | 183 mg | 4.5 mL |
105 | 231 | 105 mg | 2.6 mL | 175 mg | 4.4 mL |
100 | 220 | 100 mg | 2.5 mL | 166 mg | 4.2 mL |
95 | 209 | 95 mg | 2.4 mL | 158 mg | 4 mL |
90 | 198 | 90 mg | 2.25 mL | 150 mg | 3.75 mL |
85 | 187 | 85 mg | 2.1 mL | 141 mg | 3.5 mL |
80 | 176 | 80 mg | 2 mL | 133 mg | 3.3 mL |
75 | 165 | 75 mg | 1.9 mL | 125 mg | 3.1 mL |
70 | 154 | 70 mg | 1.75 mL | 116 mg | 2.9 mL |
65 | 143 | 65 mg | 1.6 mL | 108 mg | 2.7 mL |
60 | 132 | 60 mg | 1.5 mL | 100 mg | 2.5 mL |
55 | 121 | 55 mg | 1.4 mL | 91 mg | 2.25 mL |
50 | 110 | 50 mg | 1.25 mL | 83 mg | 2.1 mL |
45 | 99 | 45 mg | 1.1 mL | 75 mg | 1.9 mL |
40 | 88 | 40 mg | 1 mL | 66 mg | 1.6 mL |
* Applicable to all product forms except Tobramycin Injection, USP, 10 mg/mL (Pediatric). | |||||
Pediatric Patients (Greater Than 1 Week of Age):
6 to 7.5 mg/kg/day in 3 or 4 equally divided doses (2 to 2.5 mg/kg every 8 hours or 1.5 to 1.89 mg/kg every 6 hours).
Premature or Full-Term Neonates 1 Week of Age or Less: Up to 4 mg/kg/day may be administered in 2 equal doses every 12 hours.
It is desirable to limit treatment to a short term. The usual duration of treatment is 7 to 10 days. A longer course of therapy may be necessary in difficult and complicated infections. In such cases, monitoring of renal, auditory, and vestibular functions is advised, because neurotoxicity is more likely to occur when treatment is extended longer than 10 days.
Dosage in Patients with Cystic Fibrosis
In patients with cystic fibrosis, altered pharmacokinetics may result in reduced serum concentration of aminoglycosides. Measurement of tobramycin serum concentration during treatment is especially important as a basis for determining appropriate dose. In patients with severe cystic fibrosis, an initial dosing regimen of 10 mg/kg/day in 4 equally divided doses is recommended. This dosing regimen is suggested only as a guide. The serum levels of tobramycin should be measured directly during treatment due to a wide interpatient variability.
Administration for Patients with Impaired Renal Function
Whenever possible, serum tobramycin concentrations should be monitored during therapy.
Following a loading dose of 1 mg/kg, subsequent dosage in these patients must be adjusted, either with reduced doses administered at 8-hour intervals or with normal doses given at prolonged intervals. Both of these methods are suggested as guides to be used when serum levels of tobramycin cannot be measured directly. They are based on either the creatinine clearance or the serum creatinine of the patient, because these values correlate with the half-life of tobramycin. The dosage schedules derived from either method should be used in conjunction with careful clinical and laboratory observations of the patient and should be modified as necessary. Neither method should be used when dialysis is being performed.
Reduced Dosage at 8-hour Intervals
When the creatinine clearance rate is 70 mL or less per minute or when the serum creatinine value is known, the amount of the reduced dose can be determined by multiplying the normal dose from Table 1 by the percent of normal dose from the accompanying nomogram.
An alternate rough guide for determining reduced dosage at 8-hour intervals (for patients whose steady-state serum creatinine values are known) is to divide the normally recommended dose by the patient's serum creatinine.
Normal Dosage at Prolonged Intervals
If the creatinine clearance rate is not available and the patient's condition is stable, a dosage frequency in hours for the dosage given in Table 1 can be determined by multiplying the patient's serum creatinine by 6.
Dosage in Obese Patients
The appropriate dose may be calculated by using the patient's estimated lean body weight plus 40% of the excess as the basic weight on which to figure mg/kg.
Intramuscular Administration
Tobramycin Injection, USP may be administered by withdrawing the appropriate dose directly from a vial. Tobramycin Sulfate in 0.9% Sodium Chloride is not intended for intramuscular administration.
Intravenous Administration
For intravenous administration, the usual volume of diluent (0.9% Sodium Chloride Injection or 5% Dextrose Injection) is 50 to 100 mL for adult doses. For pediatric patients, the volume of diluent should be proportionately less than for adults. The diluted solution usually should be infused over a period of 20 to 60 minutes. Infusion periods of less than 20 minutes are not recommended because peak serum levels may exceed 12 mcg/mL (see WARNINGS box).
Tobramycin Injection, USP should not be physically premixed with other drugs but should be administered separately according to the recommended dose and route.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.