SUTENT® Description
(sunitinib malate)
11 DESCRIPTION
Sunitinib is a kinase inhibitor present in SUTENT capsules as the malate salt. Sunitinib malate is described chemically as (2S)-2-hydroxybutanedoic acid with N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidine)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (1:1). The molecular formula is C22H27FN4O2 ∙ C4H6O5 and the molecular weight is 532.6 Daltons. The chemical structure of sunitinib malate is:
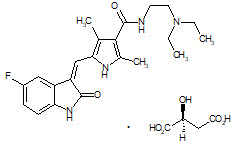
Sunitinib malate is a yellow to orange powder with a pKa of 8.95. The solubility of sunitinib malate in aqueous media over the range pH 1.2 to pH 6.8 is in excess of 25 mg/mL. The log of the distribution coefficient (octanol/water) at pH 7 is 5.2.
SUTENT (sunitinib malate) capsules are supplied as printed hard shell capsules containing 12.5 mg, 25 mg, 37.5 mg or 50 mg of sunitinib (equivalent to 16.7 mg, 33.4 mg, 50.1 mg, or 66.8 mg of sunitinib malate, respectively). The capsules contain the following inactive ingredients: croscarmellose sodium, magnesium stearate, mannitol, and povidone (K-25). The orange gelatin capsule shells contain titanium dioxide and red iron oxide; the caramel gelatin capsule shells contain titanium dioxide, red iron oxide, yellow iron oxide, and black iron oxide; and the yellow gelatin capsule shells contain titanium dioxide and yellow iron oxide. The white printing ink contains shellac, propylene glycol, sodium hydroxide, povidone, and titanium dioxide and the black printing ink contains shellac, propylene glycol, potassium hydroxide, and black iron oxide.
Find SUTENT® medical information:
Find SUTENT® medical information:
SUTENT® Quick Finder
Health Professional Information
Description
11 DESCRIPTION
Sunitinib is a kinase inhibitor present in SUTENT capsules as the malate salt. Sunitinib malate is described chemically as (2S)-2-hydroxybutanedoic acid with N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidine)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (1:1). The molecular formula is C22H27FN4O2 ∙ C4H6O5 and the molecular weight is 532.6 Daltons. The chemical structure of sunitinib malate is:

Sunitinib malate is a yellow to orange powder with a pKa of 8.95. The solubility of sunitinib malate in aqueous media over the range pH 1.2 to pH 6.8 is in excess of 25 mg/mL. The log of the distribution coefficient (octanol/water) at pH 7 is 5.2.
SUTENT (sunitinib malate) capsules are supplied as printed hard shell capsules containing 12.5 mg, 25 mg, 37.5 mg or 50 mg of sunitinib (equivalent to 16.7 mg, 33.4 mg, 50.1 mg, or 66.8 mg of sunitinib malate, respectively). The capsules contain the following inactive ingredients: croscarmellose sodium, magnesium stearate, mannitol, and povidone (K-25). The orange gelatin capsule shells contain titanium dioxide and red iron oxide; the caramel gelatin capsule shells contain titanium dioxide, red iron oxide, yellow iron oxide, and black iron oxide; and the yellow gelatin capsule shells contain titanium dioxide and yellow iron oxide. The white printing ink contains shellac, propylene glycol, sodium hydroxide, povidone, and titanium dioxide and the black printing ink contains shellac, propylene glycol, potassium hydroxide, and black iron oxide.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.