PROPOFOL (CIVICA) Clinical Pharmacology
(propofol injectable emulsion)
12 CLINICAL PHARMACOLOGY
Propofol injectable emulsion is an intravenous general anesthetic and sedation drug for use in the induction and maintenance of anesthesia or sedation. Intravenous injection of a therapeutic dose of propofol induces anesthesia, with minimal excitation, usually within 40 seconds from the start of injection (the time for one arm-brain circulation). As with other rapidly acting intravenous anesthetic agents, the half-time of the blood-brain equilibration is approximately 1 to 3 minutes, accounting for the rate of induction of anesthesia.
12.1 Mechanism of Action
The mechanism of action, like all general anesthetics, is poorly understood. However, propofol is thought to produce its sedative/anesthetic effects by the positive modulation of the inhibitory function of the neurotransmitter GABA through the ligand-gated GABAA receptors.
12.2 Pharmacodynamics
Pharmacodynamic properties of propofol are dependent upon the therapeutic blood propofol concentrations. Steady-state propofol blood concentrations are generally proportional to infusion rates. Undesirable side effects, such as cardiorespiratory depression, are likely to occur at higher blood concentrations which result from bolus dosing or rapid increases in infusion rates. An adequate interval (3 to 5 minutes) must be allowed between dose adjustments in order to assess clinical effects.
The hemodynamic effects of propofol injectable emulsion during induction of anesthesia vary. If spontaneous ventilation is maintained, the major cardiovascular effect is arterial hypotension (sometimes greater than a 30% decrease) with little or no change in heart rate and no appreciable decrease in cardiac output. If ventilation is assisted or controlled (positive pressure ventilation), there is an increase in the incidence and the degree of depression of cardiac output. Addition of an opioid, used as a premedicant, further decreases cardiac output and respiratory drive.
If anesthesia is continued by infusion of propofol injectable emulsion, the stimulation of endotracheal intubation and surgery may return arterial pressure towards normal. However, cardiac output may remain depressed. Comparative clinical studies have shown that the hemodynamic effects of propofol injectable emulsion during induction of anesthesia are generally more pronounced than with other intravenous induction agents.
Induction of anesthesia with propofol injectable emulsion is frequently associated with apnea in both adults and pediatric patients. In adult patients who received propofol injectable emulsion (2 mg/kg to 2.5 mg/kg), apnea lasted less than 30 seconds in 7% of patients, 30 seconds to 60 seconds in 24% of patients, and more than 60 seconds in 12% of patients. In pediatric patients from birth through 16 years of age assessable for apnea who received bolus doses of propofol injectable emulsion (1 mg/kg to 3.6 mg/kg), apnea lasted less than 30 seconds in 12% of patients, 30 seconds to 60 seconds in 10% of patients, and more than 60 seconds in 5% of patients.
During maintenance of general anesthesia, propofol injectable emulsion causes a decrease in spontaneous minute ventilation usually associated with an increase in carbon dioxide tension which may be marked depending upon the rate of administration and concurrent use of other medications (e.g., opioids, sedatives, etc.).
During monitored anesthesia care (MAC) sedation, attention must be given to the cardiorespiratory effects of propofol injectable emulsion. Hypotension, oxyhemoglobin desaturation, apnea, and airway obstruction can occur, especially following a rapid bolus of propofol injectable emulsion. During initiation of MAC sedation, slow infusion or slow injection techniques are preferable over rapid bolus administration. During maintenance of MAC sedation, a variable rate infusion is preferable over intermittent bolus administration in order to minimize undesirable cardiorespiratory effects. In the elderly, debilitated, or ASA-PS III or IV patients, rapid (single or repeated) bolus dose administration should not be used for MAC sedation [see Warnings and Precautions (5.12)].
Clinical and preclinical studies suggest that propofol injectable emulsion is rarely associated with elevation of plasma histamine levels.
Preliminary findings in patients with normal intraocular pressure indicate that propofol injectable emulsion produces a decrease in intraocular pressure which may be associated with a concomitant decrease in systemic vascular resistance.
Clinical studies indicate that propofol injectable emulsion when used in combination with hypocarbia increases cerebrovascular resistance and decreases cerebral blood flow, cerebral metabolic oxygen consumption, and intracranial pressure. Propofol injectable emulsion does not affect cerebrovascular reactivity to changes in arterial carbon dioxide tension [see Clinical Studies (14.2)].
Clinical studies indicate that propofol injectable emulsion does not suppress the adrenal response to ACTH.
Animal studies and limited experience in susceptible patients have not indicated any propensity of propofol injectable emulsion to induce malignant hyperthermia.
Hemosiderin deposits have been observed in the livers of dogs receiving propofol injectable emulsion containing 0.005% disodium edetate over a four-week period; the clinical significance of this is unknown.
12.3 Pharmacokinetics
The pharmacokinetics of propofol are well described by a three- compartment linear model with compartments representing the plasma, rapidly equilibrating tissues, and slowly equilibrating tissues.
Following an intravenous bolus dose, there is rapid equilibration between the plasma and the brain, accounting for the rapid onset of anesthesia. Plasma levels initially decline rapidly as a result of both distribution and metabolic clearance. Distribution accounts for about half of this decline following a bolus of propofol. However, distribution is not constant over time, but decreases as body tissues equilibrate with plasma and become saturated. The rate at which equilibration occurs is a function of the rate and duration of the infusion. When equilibration occurs there is no longer a net transfer of propofol between tissues and plasma.
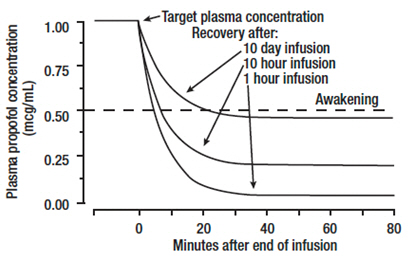
Discontinuation of the recommended doses of propofol injectable emulsion after the maintenance of anesthesia for approximately one hour, or for sedation in the ICU for one day, results in a prompt decrease in blood propofol concentrations and rapid awakening. Longer infusions (10 days of ICU sedation) result in accumulation of significant tissue stores of propofol, such that the reduction in circulating propofol is slowed and the time to awakening is increased.
By daily titration of propofol injectable emulsion dosage to achieve only the minimum effective therapeutic concentration, rapid awakening within 10 to 15 minutes can occur even after long-term administration. If, however, higher than necessary infusion levels have been maintained for a long time, propofol redistribution from fat and muscle to the plasma can be significant and slow recovery.
The figure below illustrates the fall of plasma propofol levels following infusions of various durations to provide ICU sedation.
The large contribution of distribution (about 50%) to the fall of propofol plasma levels following brief infusions means that after very long infusions a reduction in the infusion rate is appropriate by as much as half the initial infusion rate in order to maintain a constant plasma level. Therefore, failure to reduce the infusion rate in patients receiving propofol injectable emulsion for extended periods may result in excessively high blood concentrations of the drug. Thus, titration to clinical response and daily evaluation of sedation levels are important during use of propofol injectable emulsion infusion for ICU sedation.
Adults
Propofol clearance ranges from 23 mL/kg/min to 50 mL/kg/min (1.6 L/min to 3.4 L/min in 70 kg adults). It is chiefly eliminated by hepatic conjugation to inactive metabolites which are excreted by the kidney. A glucuronide conjugate accounts for about 50% of the administered dose. Propofol has a steady-state volume of distribution (10-day infusion) approaching 60 L/kg in healthy adults. A difference in pharmacokinetics due to gender has not been observed. The terminal half-life of propofol after a 10-day infusion is 1 day to 3 days.
Geriatrics
With increasing patient age, the dose of propofol needed to achieve a defined anesthetic end point (dose-requirement) decreases. This does not appear to be an age-related change in pharmacodynamics or brain sensitivity, as measured by EEG burst suppression. With increasing patient age, pharmacokinetic changes are such that, for a given intravenous bolus dose, higher peak plasma concentrations occur, which can explain the decreased dose requirement. These higher peak plasma concentrations in the elderly can predispose patients to cardiorespiratory effects including hypotension, apnea, airway obstruction, and/or arterial oxygen desaturation. The higher plasma levels reflect an age-related decrease in volume of distribution and intercompartmental clearance. Lower doses are therefore recommended for initiation and maintenance of sedation and anesthesia in elderly patients [see Dosage and Administration (2)].
Pediatrics
The pharmacokinetics of propofol were studied in children between 3 years and 12 years of age who received propofol injectable emulsion for periods of approximately 1 to 2 hours. The observed distribution and clearance of propofol in these children were similar to adults.
Organ Failure
The pharmacokinetics of propofol do not appear to be different in people with chronic hepatic cirrhosis or chronic renal impairment compared to adults with normal hepatic and renal function. The effects of acute hepatic or renal failure on the pharmacokinetics of propofol have not been studied [see Specific Populations (8.6) and (8.7)].
Find PROPOFOL (CIVICA) medical information:
Find PROPOFOL (CIVICA) medical information:
PROPOFOL (CIVICA) Quick Finder
Health Professional Information
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
Propofol injectable emulsion is an intravenous general anesthetic and sedation drug for use in the induction and maintenance of anesthesia or sedation. Intravenous injection of a therapeutic dose of propofol induces anesthesia, with minimal excitation, usually within 40 seconds from the start of injection (the time for one arm-brain circulation). As with other rapidly acting intravenous anesthetic agents, the half-time of the blood-brain equilibration is approximately 1 to 3 minutes, accounting for the rate of induction of anesthesia.
12.1 Mechanism of Action
The mechanism of action, like all general anesthetics, is poorly understood. However, propofol is thought to produce its sedative/anesthetic effects by the positive modulation of the inhibitory function of the neurotransmitter GABA through the ligand-gated GABAA receptors.
12.2 Pharmacodynamics
Pharmacodynamic properties of propofol are dependent upon the therapeutic blood propofol concentrations. Steady-state propofol blood concentrations are generally proportional to infusion rates. Undesirable side effects, such as cardiorespiratory depression, are likely to occur at higher blood concentrations which result from bolus dosing or rapid increases in infusion rates. An adequate interval (3 to 5 minutes) must be allowed between dose adjustments in order to assess clinical effects.
The hemodynamic effects of propofol injectable emulsion during induction of anesthesia vary. If spontaneous ventilation is maintained, the major cardiovascular effect is arterial hypotension (sometimes greater than a 30% decrease) with little or no change in heart rate and no appreciable decrease in cardiac output. If ventilation is assisted or controlled (positive pressure ventilation), there is an increase in the incidence and the degree of depression of cardiac output. Addition of an opioid, used as a premedicant, further decreases cardiac output and respiratory drive.
If anesthesia is continued by infusion of propofol injectable emulsion, the stimulation of endotracheal intubation and surgery may return arterial pressure towards normal. However, cardiac output may remain depressed. Comparative clinical studies have shown that the hemodynamic effects of propofol injectable emulsion during induction of anesthesia are generally more pronounced than with other intravenous induction agents.
Induction of anesthesia with propofol injectable emulsion is frequently associated with apnea in both adults and pediatric patients. In adult patients who received propofol injectable emulsion (2 mg/kg to 2.5 mg/kg), apnea lasted less than 30 seconds in 7% of patients, 30 seconds to 60 seconds in 24% of patients, and more than 60 seconds in 12% of patients. In pediatric patients from birth through 16 years of age assessable for apnea who received bolus doses of propofol injectable emulsion (1 mg/kg to 3.6 mg/kg), apnea lasted less than 30 seconds in 12% of patients, 30 seconds to 60 seconds in 10% of patients, and more than 60 seconds in 5% of patients.
During maintenance of general anesthesia, propofol injectable emulsion causes a decrease in spontaneous minute ventilation usually associated with an increase in carbon dioxide tension which may be marked depending upon the rate of administration and concurrent use of other medications (e.g., opioids, sedatives, etc.).
During monitored anesthesia care (MAC) sedation, attention must be given to the cardiorespiratory effects of propofol injectable emulsion. Hypotension, oxyhemoglobin desaturation, apnea, and airway obstruction can occur, especially following a rapid bolus of propofol injectable emulsion. During initiation of MAC sedation, slow infusion or slow injection techniques are preferable over rapid bolus administration. During maintenance of MAC sedation, a variable rate infusion is preferable over intermittent bolus administration in order to minimize undesirable cardiorespiratory effects. In the elderly, debilitated, or ASA-PS III or IV patients, rapid (single or repeated) bolus dose administration should not be used for MAC sedation [see Warnings and Precautions (5.12)].
Clinical and preclinical studies suggest that propofol injectable emulsion is rarely associated with elevation of plasma histamine levels.
Preliminary findings in patients with normal intraocular pressure indicate that propofol injectable emulsion produces a decrease in intraocular pressure which may be associated with a concomitant decrease in systemic vascular resistance.
Clinical studies indicate that propofol injectable emulsion when used in combination with hypocarbia increases cerebrovascular resistance and decreases cerebral blood flow, cerebral metabolic oxygen consumption, and intracranial pressure. Propofol injectable emulsion does not affect cerebrovascular reactivity to changes in arterial carbon dioxide tension [see Clinical Studies (14.2)].
Clinical studies indicate that propofol injectable emulsion does not suppress the adrenal response to ACTH.
Animal studies and limited experience in susceptible patients have not indicated any propensity of propofol injectable emulsion to induce malignant hyperthermia.
Hemosiderin deposits have been observed in the livers of dogs receiving propofol injectable emulsion containing 0.005% disodium edetate over a four-week period; the clinical significance of this is unknown.
12.3 Pharmacokinetics
The pharmacokinetics of propofol are well described by a three- compartment linear model with compartments representing the plasma, rapidly equilibrating tissues, and slowly equilibrating tissues.
Following an intravenous bolus dose, there is rapid equilibration between the plasma and the brain, accounting for the rapid onset of anesthesia. Plasma levels initially decline rapidly as a result of both distribution and metabolic clearance. Distribution accounts for about half of this decline following a bolus of propofol. However, distribution is not constant over time, but decreases as body tissues equilibrate with plasma and become saturated. The rate at which equilibration occurs is a function of the rate and duration of the infusion. When equilibration occurs there is no longer a net transfer of propofol between tissues and plasma.
Discontinuation of the recommended doses of propofol injectable emulsion after the maintenance of anesthesia for approximately one hour, or for sedation in the ICU for one day, results in a prompt decrease in blood propofol concentrations and rapid awakening. Longer infusions (10 days of ICU sedation) result in accumulation of significant tissue stores of propofol, such that the reduction in circulating propofol is slowed and the time to awakening is increased.
By daily titration of propofol injectable emulsion dosage to achieve only the minimum effective therapeutic concentration, rapid awakening within 10 to 15 minutes can occur even after long-term administration. If, however, higher than necessary infusion levels have been maintained for a long time, propofol redistribution from fat and muscle to the plasma can be significant and slow recovery.
The figure below illustrates the fall of plasma propofol levels following infusions of various durations to provide ICU sedation.
The large contribution of distribution (about 50%) to the fall of propofol plasma levels following brief infusions means that after very long infusions a reduction in the infusion rate is appropriate by as much as half the initial infusion rate in order to maintain a constant plasma level. Therefore, failure to reduce the infusion rate in patients receiving propofol injectable emulsion for extended periods may result in excessively high blood concentrations of the drug. Thus, titration to clinical response and daily evaluation of sedation levels are important during use of propofol injectable emulsion infusion for ICU sedation.
Adults
Propofol clearance ranges from 23 mL/kg/min to 50 mL/kg/min (1.6 L/min to 3.4 L/min in 70 kg adults). It is chiefly eliminated by hepatic conjugation to inactive metabolites which are excreted by the kidney. A glucuronide conjugate accounts for about 50% of the administered dose. Propofol has a steady-state volume of distribution (10-day infusion) approaching 60 L/kg in healthy adults. A difference in pharmacokinetics due to gender has not been observed. The terminal half-life of propofol after a 10-day infusion is 1 day to 3 days.
Geriatrics
With increasing patient age, the dose of propofol needed to achieve a defined anesthetic end point (dose-requirement) decreases. This does not appear to be an age-related change in pharmacodynamics or brain sensitivity, as measured by EEG burst suppression. With increasing patient age, pharmacokinetic changes are such that, for a given intravenous bolus dose, higher peak plasma concentrations occur, which can explain the decreased dose requirement. These higher peak plasma concentrations in the elderly can predispose patients to cardiorespiratory effects including hypotension, apnea, airway obstruction, and/or arterial oxygen desaturation. The higher plasma levels reflect an age-related decrease in volume of distribution and intercompartmental clearance. Lower doses are therefore recommended for initiation and maintenance of sedation and anesthesia in elderly patients [see Dosage and Administration (2)].
Pediatrics
The pharmacokinetics of propofol were studied in children between 3 years and 12 years of age who received propofol injectable emulsion for periods of approximately 1 to 2 hours. The observed distribution and clearance of propofol in these children were similar to adults.
Organ Failure
The pharmacokinetics of propofol do not appear to be different in people with chronic hepatic cirrhosis or chronic renal impairment compared to adults with normal hepatic and renal function. The effects of acute hepatic or renal failure on the pharmacokinetics of propofol have not been studied [see Specific Populations (8.6) and (8.7)].
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.