procainamide Hydrochloride Injection, USP
Find procainamide Hydrochloride Injection, USP medical information:
Find procainamide Hydrochloride Injection, USP medical information:
procainamide Hydrochloride Injection, USP Quick Finder
Boxed Warning
WARNING: The prolonged administration of procainamide often leads to the development of a positive anti-nuclear antibody (ANA) test, with or without symptoms of a lupus erythematosus-like syndrome. If a positive ANA titer develops, the benefit versus risks of continued procainamide therapy should be assessed.
Indications and Usage
INDICATIONS AND USAGE
Procainamide hydrochloride injection is indicated for the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia, that, in the judgement of the physician, are life-threatening. Because of the proarrhythmic effects of procainamide, its use with lesser arrhythmias is generally not recommended. Treatment of patients with asymptomatic ventricular premature contractions should be avoided.
Initiation of procainamide treatment, as with other antiarrhythmic agents used to treat life-threatening arrhythmias, should be carried out in the hospital.
Antiarrhythmic drugs have not been shown to enhance survival in patients with ventricular arrhythmias.
Because procainamide has the potential to produce serious hematological disorders (0.5 percent) particularly leukopenia or agranulocytosis (sometimes fatal), its use should be reserved for patients in whom, in the opinion of the physician, the benefits of treatment clearly outweigh the risks. (see WARNINGS and Boxed Warning.)
Dosage and Administration
DOSAGE AND ADMINISTRATION
Procainamide Hydrochloride Injection is useful for arrhythmias which require immediate suppression and for maintenance of arrhythmia control. Intravenous therapy allows most rapid control of serious arrhythmias, including those following myocardial infarction; it should be carried out in circumstances where close observation and monitoring of the patient are possible, such as in hospital or emergency facilities. Intramuscular administration is less apt to produce temporary high plasma levels but therapeutic plasma levels are not obtained as rapidly as with intravenous administration. Oral procainamide dosage forms are preferable for less urgent arrhythmias as well as for long-term maintenance after initial parenteral PA therapy.
Intramuscular administration may be used as an alternative to the oral route for patients with less threatening arrhythmias but who are nauseated or vomiting, who are ordered to receive nothing by mouth preoperatively, or who may have malabsorptive problems. An initial daily dose of 50 mg per kg body weight may be estimated. This amount should be divided into fractional doses of one-eighth to one-quarter to be injected intramuscularly every three to six hours until oral therapy is possible. If more than three injections are given, the physician may wish to assess patient factors such as age and renal function (see below), clinical response and, if available, blood levels of PA and NAPA in adjusting further doses for that individual. For treatment of arrhythmias associated with anesthesia or surgical operation, the suggested dose is 100 to 500 mg by intramuscular injection.
Intravenous administration of Procainamide Hydrochloride Injection should be done cautiously to avoid a possible hypotensive response (see PRECAUTIONS and OVERDOSAGE). Initial arrhythmia control, under ECG monitoring, may usually be accomplished safely within a half-hour by either of the two methods which follows:
a) Direct injection into a vein or into tubing of an established infusion line should be done slowly at a rate not to exceed 50 mg per minute. It is advisable to dilute either the 100 mg/mL or the 500 mg/mL concentrations of procainamide hydrochloride prior to intravenous injection to facilitate control of dosage rate. Doses of 100 mg may be administered every 5 minutes at this rate until the arrhythmia is suppressed or until 500 mg has been administered, after which it is advisable to wait 10 minutes or longer to allow for more distribution into tissues before resuming.
b) Alternatively, a loading infusion containing 20 mg of Procainamide Hydrochloride per mL (1 g diluted to 50 mL with 5% Dextrose Injection, USP) may be administered at a constant rate of 1 mL per minute for 25 to 30 minutes to deliver 500 to 600 mg of PA. Some effects may be seen after infusion of the first 100 or 200 mg; it is unusual to require more than 600 mg to achieve satisfactory antiarrhythmic effects.
The maximum advisable dosage to be given either by repeated bolus injections or such loading infusion is 1 g.
To maintain therapeutic levels, a more dilute intravenous infusion at a concentration of 2 mg/mL is convenient (1000 mg procainamide HCl in 500 mL of 5% Dextrose Injection, USP), and may be administered at 1 to 3 mL/minute. If daily total fluid intake must be limited, a 4 mg/mL concentration (1 g of Procainamide Hydrochloride Injection in 250 mL of 5% Dextrose Injection, USP) administered at 0.5 to 1.5 mL/minute will deliver an equivalent 2 to 6 mg per minute. The amount needed in a given patient to maintain the therapeutic level should be assessed principally from the clinical response, and will depend upon the patient's weight and age, renal elimination, hepatic acetylation rate, and cardiac status, but should be adjusted for each patient based upon close observation. A maintenance infusion rate of 50 mcg/min/kg body weight to a person with a normal renal PA elimination half-time of three hours may be expected to produce a plasma level of approximately 6.5 mcg/mL.
Since the principal route for elimination of PA and NAPA is renal excretion, reduced excretion will prolong the half-life of elimination and lower the dose rate needed to maintain therapeutic levels. Advancing age reduces the renal excretion of PA and NAPA independently of reductions in creatinine clearance; compared to normal young adults, there is approximately 25 percent reduction at age 50 and 50 percent at age 75.
Intravenous therapy should be terminated if persistent conduction disturbances or hypotension develop. As soon as the patient's basic cardiac rhythm appears to be stabilized, oral antiarrhythmic maintenance therapy is preferable, if indicated and possible. A period of about three to four hours (one half-time for renal elimination, ordinarily) should elapse after the last intravenous dose before administering the first dose of Procainamide Hydrochloride tablets or capsules.
DILUTIONS AND RATES FOR INTRAVENOUS INFUSIONS* | ||||
| Final Concentration | Infusion | Procainamide | Infusion |
Initial Loading Infusion | 20 mg/mL | 50 mL | 1000 mg | 1 mL/min |
Maintenance Infusion | 2 mg/mL or 4 mg/mL | 500 mL
250 mL | 1000 mg
1000 mg | 1 to 3 mL/min
0.5 to 1.5 mL/min |
The maintenance infusion rates are calculated to deliver 2 to 6 mg per minute, depending on body weight, renal elimination rate, and steady-state plasma level needed to maintain control of the arrhythmia*. The 4 mg/mL maintenance concentration may be preferred if total infused volume must be limited. | ||||
†(All infusions should be made up to final volume with 5% Dextrose Injection, USP.) | ||||
Parenteral drug products should be examined visually for particulate matter and discoloration (see HOW SUPPLIED) prior to administration.
Contraindications
CONTRAINDICATIONS
Complete Heart Block: Procainamide should not be administered to patients with complete heart block because of its effects in suppressing nodal or ventricular pacemakers and the hazard of asystole. It may be difficult to recognize complete heart block in patients with ventricular tachycardia, but if significant slowing of ventricular rate occurs during PA treatment without evidence of A-V conduction appearing, PA should be stopped. In cases of second degree A-V block or various types of hemiblock, PA should be avoided or discontinued because of the possibility of increased severity of block, unless the ventricular rate is controlled by an electrical pacemaker.
Idiosyncratic Hypersensitivity: In patients sensitive to procaine or other ester-type local anesthetics, cross sensitivity to PA is unlikely. However, it should be borne in mind, and PA should not be used if it produces acute allergic dermatitis, asthma, or anaphylactic symptoms.
Lupus Erythematosus: An established diagnosis of systemic lupus erythematosus is a contraindication to PA therapy, since aggravation of symptoms is highly likely.
Torsades de Pointes: In the unusual ventricular arrhythmia called "les torsades de pointes" (twistings of the points), characterized by alternation of one or more ventricular premature beats in the directions of the QRS complexes on ECG in persons with prolonged Q-T and often enhanced U waves, Group 1A antiarrhythmic drugs are contraindicated. Administration of PA in such cases may aggravate this special type of ventricular extrasystole or tachycardia instead of suppressing it.
Warnings and Precautions
WARNINGS
Mortality:
In the National Heart, Lung and Blood Institute's Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multicentered, randomized, double-blind study in patients with asymptomatic non-life-threatening ventricular arrhythmias who had a myocardial infarction more than six days but less than two years previously, an excessive mortality or non-fatal cardiac arrest rate (7.7%) was seen in patients treated with encainide or flecainide compared with that seen in patients assigned to matched placebo-treated group (3.0%). The average duration of treatment with encainide or flecainide in this study was ten months.
The applicability of the CAST results to other populations (e.g., those without recent myocardial infarctions) is uncertain. Considering the known proarrhythmic properties of procainamide and the lack of evidence of improved survival for any antiarrhythmic drug in patients without life-threatening arrhythmias, the use of procainamide as well as other antiarrhythmic agents should be reserved for patients with life-threatening ventricular arrhythmias.
Blood Dyscrasias: Agranulocytosis, bone marrow depression, neutropenia, hypoplastic anemia and thrombocytopenia in patients receiving procainamide hydrochloride have been reported at a rate of approximately 0.5%. Most of these patients received procainamide within the recommended dosage range. Fatalities have occurred (with approximately 20–25 percent mortality in reported cases of agranulocytosis). Since most of these events have been noted during the first 12 weeks of therapy, it is recommended that complete blood counts including white cell, differential and platelet counts be performed at weekly intervals for the first three months of therapy, and periodically thereafter. Complete blood counts should be performed promptly if the patient develops any signs of infection (such as fever, chills, sore throat or stomatitis), bruising or bleeding. If any of these hematologic disorders are identified, procainamide therapy should be discontinued. Blood counts usually return to normal within one month of discontinuation. Caution should be used in patients with pre-existing marrow failure or cytopenia of any type. (See ADVERSE REACTIONS).
Digitalis Intoxication
Caution should be exercised in the use of procainamide in arrhythmias associated with digitalis intoxication. Procainamide can suppress digitalis-induced arrhythmias; however, if there is concomitant marked disturbance of atrioventricular conduction, additional depression of conduction and ventricular asystole or fibrillation may result. Therefore, use of procainamide should be considered only if discontinuation of digitalis, and therapy with potassium, lidocaine, or phenytoin are ineffective.
First Degree Heart Block
Caution should be exercised also if the patient exhibits or develops first degree heart block while taking PA, and dosage reduction is advised in such cases. If the block persists despite dosage reduction, continuation of PA administration must be evaluated on the basis of current benefit versus risk of increased heart block.
Predigitalization for Atrial Flutter or Fibrillation
Patients with atrial flutter or fibrillation should be cardioverted or digitalized prior to PA administration to avoid enhancement of A-V conduction which may result in ventricular rate acceleration beyond tolerable limits. Adequate digitalization reduces but does not eliminate the possibility of sudden increase in ventricular rate as the atrial rate is slowed by PA in these arrhythmias.
Congestive Heart Failure
For patients in congestive heart failure, and those with acute ischemic heart disease or cardiomyopathy, caution should be used in PA therapy, since even slight depression of myocardial contractility may further reduce cardiac output of the damaged heart.
Concurrent Other Antiarrhythmic Agents
Concurrent use of PA with other Group 1A antiarrhythmic agents such as quinidine or disopyramide may produce enhanced prolongation of conduction or depression of contractility and hypotension, especially in patients with cardiac decompensation. Such use should be reserved for patients with serious arrhythmias unresponsive to a single drug and employed only if close observation is possible.
Renal Insufficiency
Renal insufficiency may lead to accumulation of high plasma levels from conventional doses of PA, with effects similar to those of overdosage (see OVERDOSAGE), unless dosage is adjusted for the individual patient.
Myasthenia Gravis
Patients with myasthenia gravis may show worsening of symptoms from PA due to its procaine-like effect on diminishing acetylcholine release at skeletal muscle motor nerve endings, so that PA administration may be hazardous without optimal adjustment of anticholinesterase medications and other precautions.
Sulfite Sensitivity
Procainamide Hydrochloride Injection contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
PRECAUTIONS
Blood-Pressure and ECG Monitoring
Blood pressure should be monitored with the patient supine during parenteral, especially intravenous, administration of PA (see DOSAGE AND ADMINISTRATION). There is a possibility that relatively high although transient plasma levels of PA may be attained and cause hypotension before the PA can be distributed from the plasma volume to its full apparent volume of distribution which is approximately 50 times greater. Therefore, caution should be exercised to avoid overly rapid administration of PA. If the blood pressure falls 15 mm Hg or more, PA administration should be temporarily discontinued. Electrocardiographic (ECG) monitoring is advisable as well, both for observation of the progress and response of the arrhythmia under treatment, and for early detection of any tendency to excessive widening of the QRS complex, prolongation of the P-R interval, or any signs of heart block (see OVERDOSAGE). Parenteral therapy with PA should be limited to use in hospitals in which monitoring and intensive supportive care are available, or to emergency situations in which equivalent observation and treatment can be provided.
General
Immediately after initiation of PA therapy, patients should be closely observed for possible hypersensitivity reactions. In conversion of atrial fibrillation to normal sinus rhythm by any means, dislodgement of mural thrombi may lead to embolization, which should be kept in mind.
After achieving and maintaining therapeutic plasma concentrations and satisfactory electrocardiographic and clinical responses, continued frequent periodic monitoring of vital signs and electrocardiograms is advised. If evidence of QRS widening of more than 25 percent or marked prolongation of the Q-T interval occurs, concern for overdosage is appropriate, and interruption of the PA infusion is advisable if a 50 percent increase occurs. Elevated serum creatinine or urea nitrogen, reduced creatinine clearance or history of renal insufficiency, as well as use in older patients (over age 50), provide grounds to anticipate that less than the usual dosage or infusion rate may suffice, since the urinary elimination of PA and NAPA may be reduced, leading to gradual accumulation beyond normally-predicted amounts. If facilities are available for measurement of plasma PA and NAPA, or acetylation capability, individual dose adjustment for optimal therapeutic levels may be easier, but close observation of clinical effectiveness is the most important criterion.
Information for Patients
The patient should be encouraged to disclose any past history of drug sensitivity, especially to procaine or other local anesthetic agents, or aspirin, and to report any history of kidney disease, congestive heart failure, myasthenia gravis, liver disease, or lupus erythematosus.
The patient should be counseled to report any symptoms of arthralgia, myalgia, fever, chills, skin rash, easy bruising, sore throat or sore mouth, infections, dark urine or icterus, wheezing, muscular weakness, chest or abdominal pain, palpitations, nausea, vomiting, anorexia, diarrhea, hallucinations, dizziness, or depression.
Laboratory Tests
Laboratory tests such as complete blood count (CBC), electrocardiogram and serum creatinine or urea nitrogen may be indicated depending on the clinical situation, and periodic rechecking of the CBC and ANA may be helpful in early detection of untoward reactions.
Drug Interactions
If other antiarrhythmic drugs are being used, additive effects on the heart may occur with PA administration, and dosage reduction may be necessary (see WARNINGS).
Anticholinergic drugs administered concurrently with PA may produce additive antivagal effects on A-V nodal conduction, although this is not as well documented for PA as for quinidine.
Patients taking PA who require neuromuscular blocking agents such as succinylcholine may require less than usual doses of the latter, due to PA effects on reducing acetylcholine release.
Drug/Laboratory Test Interactions
Suprapharmacologic concentrations of lidocaine and meprobamate may inhibit fluorescence of PA and NAPA, and propranolol shows a native fluorescence close to the PA/NAPA peak wavelengths, so that tests which depend on fluorescence measurement may be affected.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed.
Teratogenic Effects
Animal reproduction studies have not been conducted with PA. It also is not known whether PA can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. PA should be given to a pregnant woman only if clearly needed.
Adverse Reactions
ADVERSE REACTIONS
Cardiovascular System: Hypotension and serious disturbances of cardiorhythm such as ventricular asystole or fibrillation are more common with intravenous administration of PA than with intramuscular administration. Because PA is a peripheral vasodilator in concentrations higher than the usual therapeutic range, transient high plasma levels which may occur especially during intravenous administration may produce temporary but at times severe lowering of blood pressure (see OVERDOSAGE and PRECAUTIONS).
Multisystem: A lupus erythematosus-like syndrome of arthralgia, pleural or abdominal pain, and sometimes arthritis, pleural effusion, pericarditis, fever, chills, myalgia, and possibly related hematologic or skin lesions (see below) is fairly common after prolonged PA administration, perhaps more often in patients who are slow acetylators (See Boxed Warning and PRECAUTIONS). While some series have reported less than 1 in 500, others have reported the syndrome in up to 30 percent of patients on long term oral PA therapy. If discontinuation of PA does not reverse the lupoid symptoms, corticosteroid treatment may be effective.
Hematologic: Neutropenia, thrombocytopenia, or hemolytic anemia may rarely be encountered. Agranulocytosis has occurred after repeated use of PA, and deaths have been reported. (See Boxed Warning, WARNINGS section.)
Skin: Angioneurotic edema, urticaria, pruritus, flushing, and maculopapular rash have also occurred.
Gastrointestinal System: Anorexia, nausea, vomiting, abdominal pain, diarrhea or bitter taste may occur in 3 to 4 percent of patients taking oral procainamide.
Nervous System: Dizziness or giddiness, weakness, mental depression and psychosis with hallucinations have been reported.
Elevated Liver Enzymes: Elevations of transaminase with and without elevations of alkaline phosphatase and bilirubin have been reported. Some patients have had clinical symptoms (e.g., malaise, right upper quadrant pain). Deaths from liver failure have been reported.
Overdosage
OVERDOSAGE
Progressive widening of the QRS complex, prolonged Q-T and P-R intervals, lowering of the R and T waves, as well as increasing A-V block, may be seen with doses which are excessive for a given patient. Increased ventricular extrasystoles, or even ventricular tachycardia or fibrillation may occur. After intravenous administration but seldom after oral therapy, transient high plasma levels of PA may induce hypotension, affecting systolic more than diastolic pressures, especially in hypertensive patients. Such high levels may also produce central nervous depression, tremor, and even respiratory depression.
Plasma levels above 10 mcg/mL are increasingly associated with toxic findings, which are seen occasionally in the 10 to 12 mcg/mL range, more often in the 12 to 15 mcg/mL range, and commonly in patients with plasma levels greater than 15 mcg/mL.
Treatment of overdosage or toxic manifestations includes general supportive measures, close observation, monitoring of vital signs and possibly intravenous pressor agents and mechanical cardiorespiratory support. If available, PA and NAPA plasma levels may be helpful in assessing the potential degree of toxicity and response to therapy. Both PA and NAPA are removed from the circulation by hemodialysis but not peritoneal dialysis. No specific antidote for PA is known.
Description
DESCRIPTION
Procainamide Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of procainamide hydrochloride in water for injection. Each milliliter of the 2 mL vial contains procainamide hydrochloride 500 mg; methylparaben 1 mg and sodium metabisulfite 1.8 mg added in water for injection. Each milliliter of the 10 mL vial contains procainamide hydrochloride 100 mg; methylparaben 1 mg and sodium metabisulfite 0.8 mg added in water for injection. In both formulations, the solution may contain hydrochloric acid and/or sodium hydroxide for pH adjustment. pH 5.0 (4.0 to 6.0). Headspace nitrogen gassed.
Procainamide Hydrochloride Injection is intended for intravenous or intramuscular administration.
Procainamide hydrochloride, a Group 1A cardiac antiarrhythmic drug, is ρ-amino-N-[2-(diethylamino) ethyl] benzamide mono-hydrochloride. It has the following structural formula:
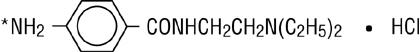
M.W. 271.79
*(locus for acetylation to N-acetyl procainamide).
It differs from procaine which is the p-aminobenzoyl ester of 2-(diethylamino)-ethanol. Procainamide as the free base has a pKa of 9.23; the monohydrochloride is very soluble in water.
Clinical Pharmacology
CLINICAL PHARMACOLOGY
Procainamide (PA) increases the effective refractory period of the atria, and to a lesser extent the bundle of His-Purkinje system and ventricles of the heart. It reduces impulse conduction velocity in the atria, His-Purkinje fibers, and ventricular muscle, but has variable effects on the atrioventricular (A-V) node, a direct slowing action and a weaker vagolytic effect which may speed A-V conduction slightly. Myocardial excitability is reduced in the atria, Purkinje fibers, papillary muscles, and ventricles by an increase in the threshold for excitation, combined with inhibition of ectopic pacemaker activity by retardation of the slow phase of diastolic depolarization, thus decreasing automaticity especially in ectopic sites. Contractility of the undamaged heart is usually not affected by therapeutic concentrations, although slight reduction of cardiac output may occur, and may be significant in the presence of myocardial damage. Therapeutic levels of PA may exert vagolytic effects and produce slight acceleration of heart rate, while high or toxic concentrations may prolong A-V conduction time or induce A-V block, or even cause abnormal automaticity and spontaneous firing by unknown mechanisms.
The electrocardiogram may reflect these effects by showing slight sinus tachycardia (due to the anticholinergic action) and widened QRS complexes and, less regularly, prolonged Q-T and P-R intervals (due to longer systole and slower conduction), as well as some decrease in QRS and T wave amplitude. These direct effects of PA on electrical activity, conduction, responsiveness, excitability and automaticity are characteristic of a Group 1A antiarrhythmic agent, the prototype for which is quinidine; PA effects are very similar. However, PA has weaker vagal blocking action than does quinidine, does not induce alpha-adrenergic blockade, and is less depressing to cardiac contractility.
Following intramuscular injection, procainamide is rapidly absorbed into the bloodstream, and plasma levels peak in 15 to 60 minutes, considerably faster than orally administered procainamide hydrochloride tablets or capsules which produce peak plasma levels in 90 to 120 minutes. Intravenous administration of Procainamide Hydrochloride Injection can produce therapeutic procainamide levels within minutes after infusion is started. About 15 to 20 percent of PA is reversibly bound to plasma proteins, and considerable amounts are more slowly and reversibly bound to tissues of the heart, liver, lung, and kidney. The apparent volume of distribution eventually reaches about 2 liters per kilogram body weight with a half-time of approximately five minutes. While PA has been shown in the dog to cross the blood-brain barrier, it did not concentrate in the brain at levels higher than in plasma. It is not known if PA crosses the placenta. Plasma esterases are far less active in hydrolysis of PA than of procaine. The half-time for elimination of PA is three to four hours in patients with normal renal function, but reduced creatinine clearance and advancing age each prolong the half-time of elimination of PA.
A significant fraction of the circulating PA may be metabolized in hepatocytes to N-acetylprocainamide (NAPA), ranging from 16 to 21 percent of an administered dose in "slow acetylators" to 24 to 33 percent in "fast-acetylators". Since NAPA also has significant antiarrhythmic activity and somewhat slower renal clearance than PA, both hepatic acetylation rate capability and renal function, as well as age, have significant effects on the effective biologic half-time of therapeutic action of administered PA and the NAPA derivative. Trace amounts may be excreted in the urine as free and conjugated ρ-aminobenzoic acid, 30 to 60 percent as unchanged PA, and 6 to 52 percent as the NAPA derivative. Both PA and NAPA are eliminated by active tubular secretion as well as by glomerular filtration. Action of PA on the central nervous system is not prominent, but high plasma concentrations may cause tremors. While therapeutic plasma levels for PA have been reported to be 3 to 10 mcg/mL certain patients such as those with sustained ventricular tachycardia, may need higher levels for adequate control. This may justify the increased risk of toxicity (see OVERDOSAGE). Where programmed ventricular stimulation has been used to evaluate efficacy of PA in preventing recurrent ventricular tachyarrhythmias, higher plasma levels (mean, 13.6 mcg/mL) of PA were found necessary for adequate control.
How Supplied/Storage and Handling
HOW SUPPLIED
Procainamide Hydrochloride Injection, USP is available in multiple-dose 10 mL vials providing 100 mg procainamide hydrochloride per mL and 2 mL vials providing 500 mg procainamide hydrochloride per mL.
| Unit of Sale | Concentration |
|---|---|
| NDC 0409-1902-01 Case containing 25 cartons of 1 multiple-dose vial | 1 g/10 mL (100 mg/mL) |
| NDC 0409-1903-01 Bundle containing 25 cartons of 1 multiple-dose vial | 1 g/2 mL (500 mg/mL) |
The solutions, which are clear and colorless initially, may develop a slightly yellow color in time. This does not indicate a change which should preclude its use, but a solution any darker than light amber or otherwise discolored should not be used.
Store at 20°C to 25°C (68°F to 77°F). [See USP Controlled Room Temperature.]
Distributed by Hospira Inc., Lake Forest, IL 60045 USA

LAB-1321-3.0
Revised: 03/2021
Other
Resources
Didn’t find what you were looking for?
Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this product, click the link below to submit your information: Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer product, please call Pfizer Medical Information at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800)-332-1088.