pemetrexed disodium (solution) vial Clinical Studies
(pemetrexed for injection)
14 CLINICAL STUDIES
14.1 Non-Squamous NSCLC
Initial Treatment in Combination with Cisplatin
The efficacy of pemetrexed was evaluated in Study JMDB (NCT00087711), a multi-center, randomized (1:1), open-label study conducted in 1725 chemotherapy-naive patients with Stage IIIb/IV NSCLC. Patients were randomized to receive pemetrexed with cisplatin or gemcitabine with cisplatin. Randomization was stratified by Eastern Cooperative Oncology Group Performance Status (ECOG PS 0 versus 1), gender, disease stage, basis for pathological diagnosis (histopathological/cytopathological), history of brain metastases, and investigative center. Pemetrexed was administered intravenously over 10 minutes at a dose of 500 mg/m2 on Day 1 of each 21-day cycle. Cisplatin was administered intravenously at a dose of 75 mg/m2 approximately 30 minutes after pemetrexed administration on Day 1 of each cycle, gemcitabine was administered at a dose of 1250 mg/m2 on Day 1 and Day 8, and cisplatin was administered intravenously at a dose of 75 mg/m2 approximately 30 minutes after administration of gemcitabine, on Day 1 of each 21-day cycle. Treatment was administered up to a total of 6 cycles; patients in both arms received folic acid, vitamin B12, and dexamethasone [see Dosage and Administration (2.4)]. The primary efficacy outcome measure was overall survival.
A total of 1725 patients were enrolled with 862 patients randomized to pemetrexed in combination with cisplatin and 863 patients to gemcitabine in combination with cisplatin. The median age was 61 years (range 26–83 years), 70% were male, 78% were White, 17% were Asian, 2.9% were Hispanic or Latino, and 2.1% were Black or African American, and <1% were other ethnicities. Among patients for whom ECOG PS (n=1722) and smoking history (n=1516) were collected, 65% had an ECOG PS of 1, 36% had an ECOG PS of 0, and 84% were smokers. For tumor characteristics, 73% had non-squamous NSCLC and 27% had squamous NSCLC; 76% had Stage IV disease. Among 1252 patients with non-squamous NSCLC histology, 68% had a diagnosis of adenocarcinoma, 12% had large cell histology and 20% had other histologic subtypes.
Efficacy results in Study JMDB are presented in Table 8 and Figure 1.
| Efficacy Parameter | Pemetrexed plus Cisplatin (N=862) | Gemcitabine plus Cisplatin (N=863) |
|---|---|---|
| Overall Survival | ||
| Median (months) | 10.3 | 10.3 |
| (95% CI) | (9.8–11.2) | (9.6–10.9) |
| Hazard ratio (HR)*,† | 0.94 | |
| (95% CI) | (0.84–1.05) | |
| Progression-Free Survival | ||
| Median (months) | 4.8 | 5.1 |
| (95% CI) | (4.6–5.3) | (4.6–5.5) |
| Hazard ratio (HR)*,† | 1.04 | |
| (95% CI) | (0.94–1.15) | |
| Overall Response Rate | 27.1% | 24.7% |
| (95% CI) | (24.2–30.1) | (21.8–27.6) |
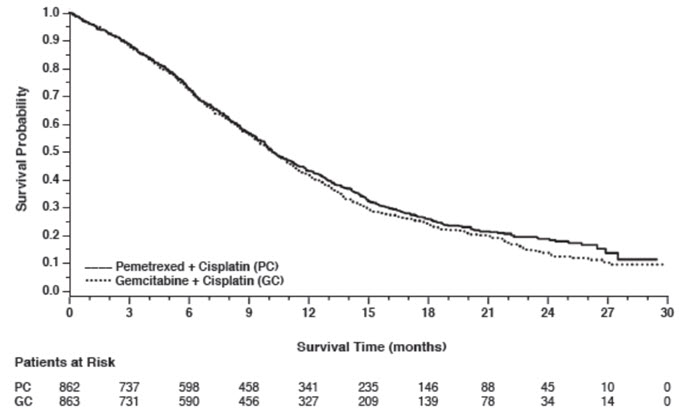
Figure 1: Kaplan-Meier Curves for Overall Survival in Study JMDB
In pre-specified analyses assessing the impact of NSCLC histology on overall survival, clinically relevant differences in survival according to histology were observed. These subgroup analyses are shown in Table 9 and Figures 2 and 3. This difference in treatment effect for pemetrexed based on histology demonstrating a lack of efficacy in squamous cell histology was also observed in Studies JMEN and JMEI.
| Histologic Subgroups | Pemetrexed plus Cisplatin (N=862) | Gemcitabine plus Cisplatin (N=863) |
|---|---|---|
| Non-squamous NSCLC (N=1252) | ||
| Median (months) | 11.0 | 10.1 |
| (95% CI) | (10.1–12.5) | (9.3–10.9) |
| HR*,† | 0.84 | |
| (95% CI) | (0.74–0.96) | |
| Adenocarcinoma (N=847) | ||
| Median (months) | 12.6 | 10.9 |
| (95% CI) | (10.7–13.6) | (10.2–11.9) |
| HR*,† | 0.84 | |
| (95% CI) | (0.71–0.99) | |
| Large Cell (N=153) | ||
| Median (months) | 10.4 | 6.7 |
| (95% CI) | (8.6–14.1) | (5.5–9.0) |
| HR*,† | 0.67 | |
| (95% CI) | (0.48–0.96) | |
| Non-squamous, not otherwise specified (N=252) | ||
| Median (months) | 8.6 | 9.2 |
| (95% CI) | (6.8–10.2) | (8.1–10.6) |
| HR*,† | 1.08 | |
| (95% CI) | (0.81–1.45) | |
| Squamous Cell (N=473) | ||
| Median (months) | 9.4 | 10.8 |
| (95% CI) | (8.4–10.2) | (9.5–12.1) |
| HR*,† | 1.23 | |
| (95% CI) | (1.00–1.51) | |
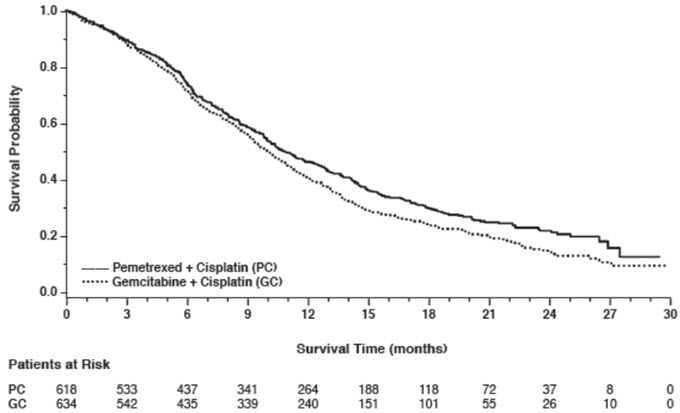
Figure 2: Kaplan-Meier Curves for Overall Survival in Non-squamous NSCLC in Study JMDB
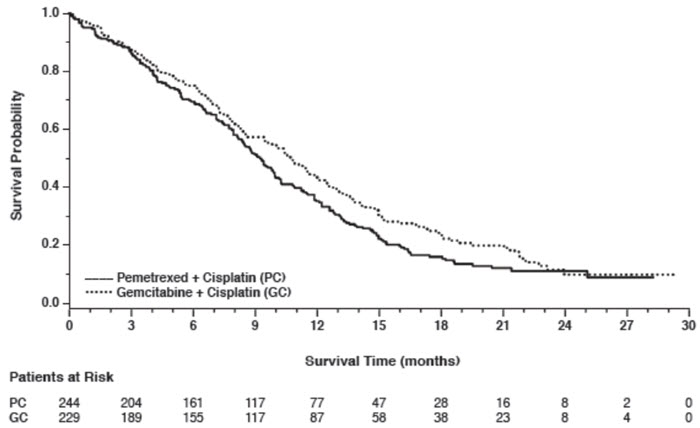
Figure 3: Kaplan-Meier Curves for Overall Survival in Squamous NSCLC in Study JMDB
Maintenance Treatment Following First-line Non-Pemetrexed Containing Platinum-Based Chemotherapy
The efficacy of pemetrexed as maintenance therapy following first-line platinum-based chemotherapy was evaluated in Study JMEN (NCT00102804), a multicenter, randomized (2:1), double-blind, placebo-controlled study conducted in 663 patients with Stage IIIb/IV NSCLC who did not progress after four cycles of platinum-based chemotherapy. Patients were randomized to receive pemetrexed 500 mg/m2 intravenously every 21 days or placebo until disease progression or intolerable toxicity. Patients in both study arms received folic acid, vitamin B12, and dexamethasone [see Dosage and Administration (2.4)]. Randomization was carried out using a minimization approach [Pocock and Simon (1975)] using the following factors: gender, ECOG PS (0 versus 1), response to prior chemotherapy (complete or partial response versus stable disease), history of brain metastases (yes versus no), non-platinum component of induction therapy (docetaxel versus gemcitabine versus paclitaxel), and disease stage (IIIb versus IV). The major efficacy outcome measures were progression-free survival based on assessment by independent review and overall survival; both were measured from the date of randomization in Study JMEN.
A total of 663 patients were enrolled with 441 patients randomized to pemetrexed and 222 patients randomized to placebo. The median age was 61 years (range 26–83 years); 73% were male; 65% were White, 32% were Asian, 2.9% were Hispanic or Latino, and <2% were other ethnicities; 60% had an ECOG PS of 1; and 73% were current or former smokers. Median time from initiation of platinum-based chemotherapy to randomization was 3.3 months (range 1.6 to 5.1 months) and 49% of the population achieved a partial or complete response to first-line, platinum-based chemotherapy. With regard to tumor characteristics, 81% had Stage IV disease, 73% had non-squamous NSCLC and 27% had squamous NSCLC. Among the 481 patients with non-squamous NSCLC, 68% had adenocarcinoma, 4% had large cell, and 28% had other histologies.
Efficacy results are presented in Table 10 and Figure 4.
| Efficacy Parameter | Pemetrexed | Placebo |
|---|---|---|
| ||
| Overall survival | N=441 | N=222 |
| Median (months) | 13.4 | 10.6 |
| (95% CI) | (11.9–15.9) | (8.7–12.0) |
| Hazard ratio* | 0.79 | |
| (95% CI) | (0.65–0.95) | |
| p-value | p=0.012 | |
| Progression-free survival per independent review | N=387 | N=194 |
| Median (months) | 4.0 | 2.0 |
| (95% CI) | (3.1–4.4) | (1.5–2.8) |
| Hazard ratio* | 0.60 | |
| (95% CI) | (0.49–0.73) | |
| p-value | p<0.00001 | |
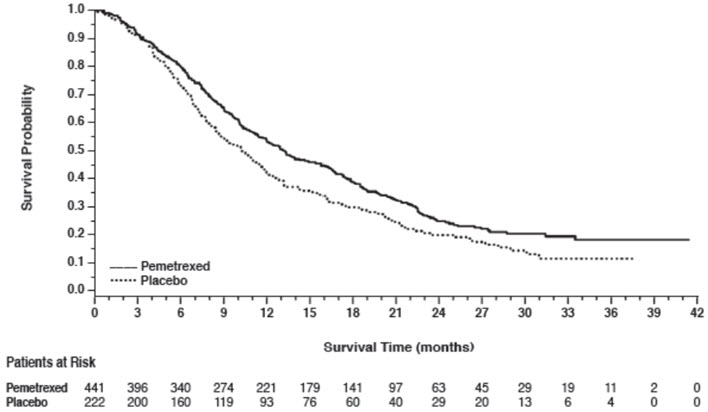
Figure 4: Kaplan-Meier Curves for Overall Survival in Study JMEN
The results of pre-specified subgroup analyses by NSCLC histology are presented in Table 11 and Figures 5 and 6.
| Efficacy Parameter | Overall Survival | Progression-Free Survival Per Independent Review | ||
|---|---|---|---|---|
| Pemetrexed (N=441) | Placebo (N=222) | Pemetrexed (N=387) | Placebo (N=194) | |
| Non-squamous NSCLC (n=481) | ||||
| Median (months) | 15.5 | 10.3 | 4.4 | 1.8 |
| HR* | 0.70 | 0.47 | ||
| (95% CI) | (0.56–0.88) | (0.37–0.60) | ||
| Adenocarcinoma (n=328) | ||||
| Median (months) | 16.8 | 11.5 | 4.6 | 2.7 |
| HR* | 0.73 | 0.51 | ||
| (95% CI) | (0.56–0.96) | (0.38–0.68) | ||
| Large cell carcinoma (n=20) | ||||
| Median (months) | 8.4 | 7.9 | 4.5 | 1.5 |
| HR* | 0.98 | 0.40 | ||
| (95% CI) | (0.36–2.65) | (0.12–1.29) | ||
| Other† (n=133) | ||||
| Median (months) | 11.3 | 7.7 | 4.1 | 1.6 |
| HR* | 0.61 | 0.44 | ||
| (95% CI) | (0.40–0.94) | (0.28–0.68) | ||
| Squamous cell NSCLC (n=182) | ||||
| Median (months) | 9.9 | 10.8 | 2.4 | 2.5 |
| HR* | 1.07 | 1.03 | ||
| (95% CI) | (0.77–1.50) | (0.71–1.49) | ||
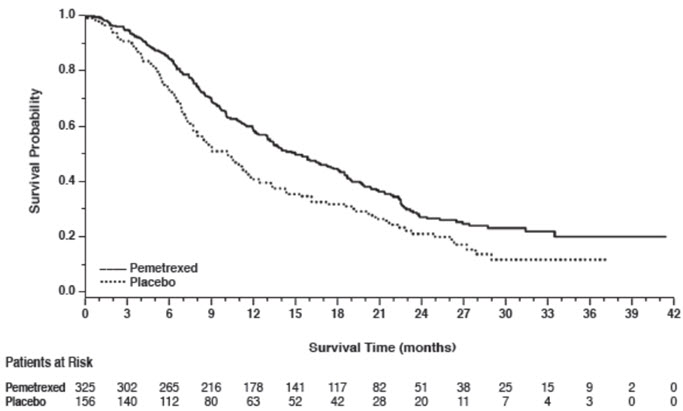
Figure 5: Kaplan-Meier Curves for Overall Survival in Non-squamous NSCLC in Study JMEN
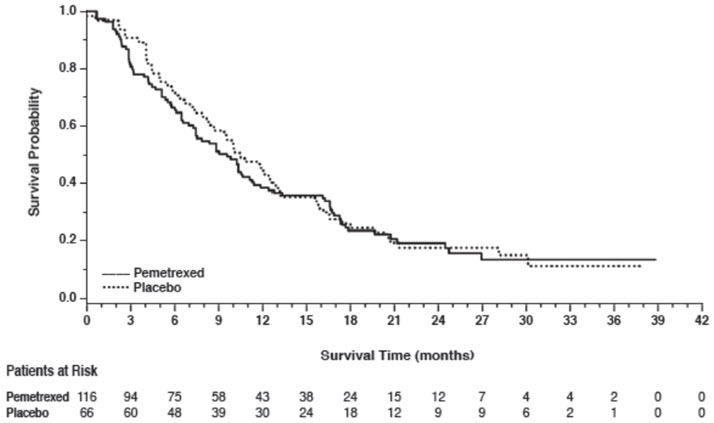
Figure 6: Kaplan-Meier Curves for Overall Survival in Squamous NSCLC in Study JMEN
Maintenance Treatment Following First-line Pemetrexed Plus Platinum Chemotherapy
The efficacy of pemetrexed as maintenance therapy following first-line platinum-based chemotherapy was also evaluated in PARAMOUNT (NCT00789373), a multi-center, randomized (2:1), double-blind, placebo-controlled study conducted in patients with Stage IIIb/IV non-squamous NSCLC who had completed four cycles of pemetrexed in combination with cisplatin and achieved a complete response (CR) or partial response (PR) or stable disease (SD). Patients were required to have an ECOG PS of 0 or 1. Patients were randomized to receive pemetrexed 500 mg/m2 intravenously every 21 days or placebo until disease progression. Randomization was stratified by response to pemetrexed in combination with cisplatin induction therapy (CR or PR versus SD), disease stage (IIIb versus IV), and ECOG PS (0 versus 1). Patients in both arms received folic acid, vitamin B12, and dexamethasone. The main efficacy outcome measure was investigator-assessed progression-free survival (PFS) and an additional efficacy outcome measure was overall survival (OS); PFS and OS were measured from the time of randomization.
A total of 539 patients were enrolled with 359 patients randomized to pemetrexed and 180 patients randomized to placebo. The median age was 61 years (range 32 to 83 years); 58% were male; 95% were White, 4.5% were Asian, and <1% were Black or African American; 67% had an ECOG PS of 1; 78% were current or former smokers; and 43% of the population achieved a partial or complete response to first-line, platinum-based chemotherapy. With regard to tumor characteristics, 91% had Stage IV disease, 87% had adenocarcinoma, 7% had large cell, and 6% had other histologies.
Efficacy results for PARAMOUNT are presented in Table 12 and Figure 7.
| Efficacy Parameter | Pemetrexed (N=359) | Placebo (N=180) |
|---|---|---|
| Overall survival | ||
| Median (months) | 13.9 | 11.0 |
| (95% CI) | (12.8–16.0) | (10.0–12.5) |
| Hazard ratio (HR)* | 0.78 | |
| (95% CI) | (0.64–0.96) | |
| p-value | p=0.02 | |
| Progression-free survival† | ||
| Median (months) | 4.1 | 2.8 |
| (95% CI) | (3.2–4.6) | (2.6–3.1) |
| Hazard ratio (HR)* | 0.62 | |
| (95% CI) | (0.49–0.79) | |
| p-value | p<0.0001 | |
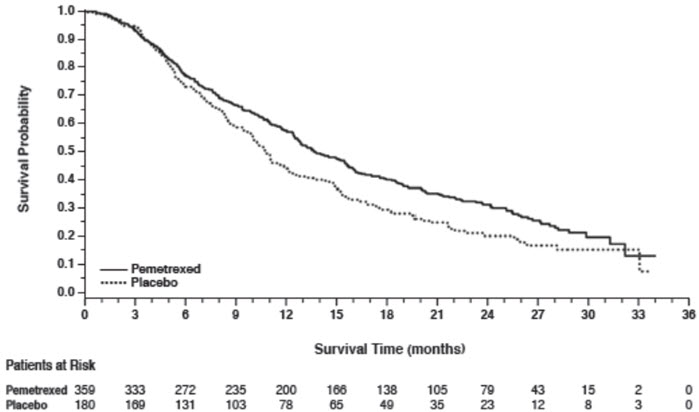
Figure 7: Kaplan-Meier Curves for Overall Survival in PARAMOUNT
Treatment of Recurrent Disease After Prior Chemotherapy
The efficacy of pemetrexed was evaluated in Study JMEI (NCT00004881), a multicenter, randomized (1:1), open-label study conducted in patients with Stage III or IV NSCLC that had recurred or progressed following one prior chemotherapy regimen for advanced disease. Patients were randomized to receive pemetrexed 500 mg/m2 intravenously or docetaxel 75 mg/m2 as a 1-hour intravenous infusion once every 21 days. Patients randomized to pemetrexed also received folic acid and vitamin B12. The study was designed to show that overall survival with pemetrexed was non-inferior to docetaxel, as the major efficacy outcome measure, and that overall survival was superior for patients randomized to pemetrexed compared to docetaxel, as a secondary outcome measure.
A total of 571 patients were enrolled with 283 patients randomized to pemetrexed and 288 patients randomized to docetaxel. The median age was 58 years (range 22 to 87 years); 72% were male; 71% were White, 24% were Asian, 2.8% were Black or African American, 1.8% were Hispanic or Latino, and <2% were other ethnicities; 88% had an ECOG PS of 0 or 1. With regard to tumor characteristics, 75% had Stage IV disease; 53% had adenocarcinoma, 30% had squamous histology; 8% large cell; and 9% had other histologic subtypes of NSCLC.
The efficacy results in the overall population and in subgroup analyses based on histologic subtype are provided in Tables 13 and 14, respectively. Study JMEI did not show an improvement in overall survival in the intent-to-treat population. In subgroup analyses, there was no evidence of a treatment effect on survival in patients with squamous NSCLC; the absence of a treatment effect in patients with NSCLC of squamous histology was also observed in Studies JMDB and JMEN [see Clinical Studies (14.1)].
| Efficacy Parameter | Pemetrexed (N=283) | Docetaxel (N=288) |
|---|---|---|
| ||
| Overall survival | ||
| Median (months) | 8.3 | 7.9 |
| (95% CI) | (7.0–9.4) | (6.3–9.2) |
| Hazard ratio* | 0.99 | |
| (95% CI) | (0.82–1.20) | |
| Progression-free survival | ||
| Median (months) | 2.9 | 2.9 |
| (95% CI) | (2.4–3.1) | (2.7–3.4) |
| Hazard ratio* | 0.97 | |
| (95% CI) | (0.82–1.16) | |
| Overall response rate | 8.5% | 8.3% |
| (95% CI) | (5.2–11.7) | (5.1–11.5) |
| Histologic Subgroups | Pemetrexed (N=283) | Docetaxel (N=288) |
|---|---|---|
| Non-squamous NSCLC (N=399) | ||
| Median (months) | 9.3 | 8.0 |
| (95% CI) | (7.8–9.7) | (6.3–9.3) |
| HR* | 0.89 | |
| (95% CI) | (0.71–1.13) | |
| Adenocarcinoma (N=301) | ||
| Median (months) | 9.0 | 9.2 |
| (95% CI) | (7.6–9.6) | (7.5–11.3) |
| HR* | 1.09 | |
| (95% CI) | (0.83–1.44) | |
| Large Cell (N=47) | ||
| Median (months) | 12.8 | 4.5 |
| (95% CI) | (5.8–14.0) | (2.3–9.1) |
| HR* | 0.38 | |
| (95% CI) | (0.18–0.78) | |
| Other† (N=51) | ||
| Median (months) | 9.4 | 7.9 |
| (95% CI) | (6.0–10.1) | (4.0–8.9) |
| HR* | 0.62 | |
| (95% CI) | (0.32–1.23) | |
| Squamous NSCLC (N=172) | ||
| Median (months) | 6.2 | 7.4 |
| (95% CI) | (4.9–8.0) | (5.6–9.5) |
| HR* | 1.32 | |
| (95% CI) | (0.93–1.86) | |
14.2 Mesothelioma
The efficacy of pemetrexed was evaluated in Study JMCH (NCT00005636), a multicenter, randomized (1:1), single-blind study conducted in patients with MPM who had received no prior chemotherapy. Patients were randomized (n=456) to receive pemetrexed 500 mg/m2 intravenously over 10 minutes followed 30 minutes later by cisplatin 75 mg/m2 intravenously over two hours on Day 1 of each 21-day cycle or to receive cisplatin 75 mg/m2 intravenously over 2 hours on Day 1 of each 21-day cycle; treatment continued until disease progression or intolerable toxicity. The study was modified after randomization and treatment of 117 patients to require that all patients receive folic acid 350 mcg to 1000 mcg daily beginning 1 to 3 weeks prior to the first dose of pemetrexed and continuing until 1 to 3 weeks after the last dose, vitamin B12 1000 mcg intramuscularly 1 to 3 weeks prior to first dose of pemetrexed and every 9 weeks thereafter, and dexamethasone 4 mg orally, twice daily, for 3 days starting the day prior to each pemetrexed dose. Randomization was stratified by multiple baseline variables including KPS, histologic subtype (epithelial, mixed, sarcomatoid, other), and gender. The major efficacy outcome measure was overall survival and additional efficacy outcome measures were time to disease progression, overall response rate, and response duration.
A total of 448 patients received at least one dose of protocol-specified therapy; 226 patients were randomized to and received at least one dose of pemetrexed plus cisplatin, and 222 patients were randomized to and received cisplatin. Among the 226 patients who received cisplatin with pemetrexed, 74% received full supplementation with folic acid and vitamin B12 during study therapy, 14% were never supplemented, and 12% were partially supplemented. Across the study population, the median age was 61 years (range: 20 to 86 years); 81% were male; 92% were White, 5% were Hispanic or Latino, 3.1% were Asian, and <1% were other ethnicities; and 54% had a baseline KPS score of 90–100% and 46% had a KPS score of 70–80%. With regard to tumor characteristics, 46% had Stage IV disease, 31% Stage III, 15% Stage II, and 7% Stage I disease at baseline; the histologic subtype of mesothelioma was epithelial in 68% of patients, mixed in 16%, sarcomatoid in 10% and other histologic subtypes in 6%. The baseline demographics and tumor characteristics of the subgroup of fully supplemented patients was similar to the overall study population.
The efficacy results from Study JMCH are summarized in Table 15 and Figure 8.
| Efficacy Parameter | All Randomized and Treated Patients (N=448) | Fully Supplemented Patients (N=331) | ||
|---|---|---|---|---|
| Pemetrexed/Cisplatin (N=226) | Cisplatin (N=222) | Pemetrexed/Cisplatin (N=168) | Cisplatin (N=163) | |
| Median overall survival (months) | 12.1 | 9.3 | 13.3 | 10.0 |
| (95% CI) | (10.0–14.4) | (7.8–10.7) | (11.4–14.9) | (8.4–11.9) |
| Hazard ratio* | 0.77 | 0.75 | ||
| Log rank p-value | 0.020 | NA† | ||
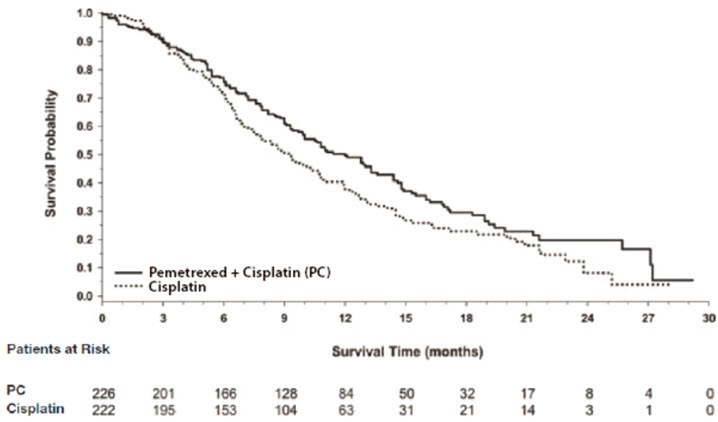
Figure 8: Kaplan-Meier Curves for Overall Survival in Study JMCH
Based upon prospectively defined criteria (modified Southwest Oncology Group methodology) the objective tumor response rate for pemetrexed plus cisplatin was greater than the objective tumor response rate for cisplatin alone. There was also improvement in lung function (forced vital capacity) in the pemetrexed plus cisplatin arm compared to the control arm.
Find pemetrexed disodium (solution) vial medical information:
Find pemetrexed disodium (solution) vial medical information:
pemetrexed disodium (solution) vial Quick Finder
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 Non-Squamous NSCLC
Initial Treatment in Combination with Cisplatin
The efficacy of pemetrexed was evaluated in Study JMDB (NCT00087711), a multi-center, randomized (1:1), open-label study conducted in 1725 chemotherapy-naive patients with Stage IIIb/IV NSCLC. Patients were randomized to receive pemetrexed with cisplatin or gemcitabine with cisplatin. Randomization was stratified by Eastern Cooperative Oncology Group Performance Status (ECOG PS 0 versus 1), gender, disease stage, basis for pathological diagnosis (histopathological/cytopathological), history of brain metastases, and investigative center. Pemetrexed was administered intravenously over 10 minutes at a dose of 500 mg/m2 on Day 1 of each 21-day cycle. Cisplatin was administered intravenously at a dose of 75 mg/m2 approximately 30 minutes after pemetrexed administration on Day 1 of each cycle, gemcitabine was administered at a dose of 1250 mg/m2 on Day 1 and Day 8, and cisplatin was administered intravenously at a dose of 75 mg/m2 approximately 30 minutes after administration of gemcitabine, on Day 1 of each 21-day cycle. Treatment was administered up to a total of 6 cycles; patients in both arms received folic acid, vitamin B12, and dexamethasone [see Dosage and Administration (2.4)]. The primary efficacy outcome measure was overall survival.
A total of 1725 patients were enrolled with 862 patients randomized to pemetrexed in combination with cisplatin and 863 patients to gemcitabine in combination with cisplatin. The median age was 61 years (range 26–83 years), 70% were male, 78% were White, 17% were Asian, 2.9% were Hispanic or Latino, and 2.1% were Black or African American, and <1% were other ethnicities. Among patients for whom ECOG PS (n=1722) and smoking history (n=1516) were collected, 65% had an ECOG PS of 1, 36% had an ECOG PS of 0, and 84% were smokers. For tumor characteristics, 73% had non-squamous NSCLC and 27% had squamous NSCLC; 76% had Stage IV disease. Among 1252 patients with non-squamous NSCLC histology, 68% had a diagnosis of adenocarcinoma, 12% had large cell histology and 20% had other histologic subtypes.
Efficacy results in Study JMDB are presented in Table 8 and Figure 1.
| Efficacy Parameter | Pemetrexed plus Cisplatin (N=862) | Gemcitabine plus Cisplatin (N=863) |
|---|---|---|
| Overall Survival | ||
| Median (months) | 10.3 | 10.3 |
| (95% CI) | (9.8–11.2) | (9.6–10.9) |
| Hazard ratio (HR)*,† | 0.94 | |
| (95% CI) | (0.84–1.05) | |
| Progression-Free Survival | ||
| Median (months) | 4.8 | 5.1 |
| (95% CI) | (4.6–5.3) | (4.6–5.5) |
| Hazard ratio (HR)*,† | 1.04 | |
| (95% CI) | (0.94–1.15) | |
| Overall Response Rate | 27.1% | 24.7% |
| (95% CI) | (24.2–30.1) | (21.8–27.6) |

Figure 1: Kaplan-Meier Curves for Overall Survival in Study JMDB
In pre-specified analyses assessing the impact of NSCLC histology on overall survival, clinically relevant differences in survival according to histology were observed. These subgroup analyses are shown in Table 9 and Figures 2 and 3. This difference in treatment effect for pemetrexed based on histology demonstrating a lack of efficacy in squamous cell histology was also observed in Studies JMEN and JMEI.
| Histologic Subgroups | Pemetrexed plus Cisplatin (N=862) | Gemcitabine plus Cisplatin (N=863) |
|---|---|---|
| Non-squamous NSCLC (N=1252) | ||
| Median (months) | 11.0 | 10.1 |
| (95% CI) | (10.1–12.5) | (9.3–10.9) |
| HR*,† | 0.84 | |
| (95% CI) | (0.74–0.96) | |
| Adenocarcinoma (N=847) | ||
| Median (months) | 12.6 | 10.9 |
| (95% CI) | (10.7–13.6) | (10.2–11.9) |
| HR*,† | 0.84 | |
| (95% CI) | (0.71–0.99) | |
| Large Cell (N=153) | ||
| Median (months) | 10.4 | 6.7 |
| (95% CI) | (8.6–14.1) | (5.5–9.0) |
| HR*,† | 0.67 | |
| (95% CI) | (0.48–0.96) | |
| Non-squamous, not otherwise specified (N=252) | ||
| Median (months) | 8.6 | 9.2 |
| (95% CI) | (6.8–10.2) | (8.1–10.6) |
| HR*,† | 1.08 | |
| (95% CI) | (0.81–1.45) | |
| Squamous Cell (N=473) | ||
| Median (months) | 9.4 | 10.8 |
| (95% CI) | (8.4–10.2) | (9.5–12.1) |
| HR*,† | 1.23 | |
| (95% CI) | (1.00–1.51) | |

Figure 2: Kaplan-Meier Curves for Overall Survival in Non-squamous NSCLC in Study JMDB

Figure 3: Kaplan-Meier Curves for Overall Survival in Squamous NSCLC in Study JMDB
Maintenance Treatment Following First-line Non-Pemetrexed Containing Platinum-Based Chemotherapy
The efficacy of pemetrexed as maintenance therapy following first-line platinum-based chemotherapy was evaluated in Study JMEN (NCT00102804), a multicenter, randomized (2:1), double-blind, placebo-controlled study conducted in 663 patients with Stage IIIb/IV NSCLC who did not progress after four cycles of platinum-based chemotherapy. Patients were randomized to receive pemetrexed 500 mg/m2 intravenously every 21 days or placebo until disease progression or intolerable toxicity. Patients in both study arms received folic acid, vitamin B12, and dexamethasone [see Dosage and Administration (2.4)]. Randomization was carried out using a minimization approach [Pocock and Simon (1975)] using the following factors: gender, ECOG PS (0 versus 1), response to prior chemotherapy (complete or partial response versus stable disease), history of brain metastases (yes versus no), non-platinum component of induction therapy (docetaxel versus gemcitabine versus paclitaxel), and disease stage (IIIb versus IV). The major efficacy outcome measures were progression-free survival based on assessment by independent review and overall survival; both were measured from the date of randomization in Study JMEN.
A total of 663 patients were enrolled with 441 patients randomized to pemetrexed and 222 patients randomized to placebo. The median age was 61 years (range 26–83 years); 73% were male; 65% were White, 32% were Asian, 2.9% were Hispanic or Latino, and <2% were other ethnicities; 60% had an ECOG PS of 1; and 73% were current or former smokers. Median time from initiation of platinum-based chemotherapy to randomization was 3.3 months (range 1.6 to 5.1 months) and 49% of the population achieved a partial or complete response to first-line, platinum-based chemotherapy. With regard to tumor characteristics, 81% had Stage IV disease, 73% had non-squamous NSCLC and 27% had squamous NSCLC. Among the 481 patients with non-squamous NSCLC, 68% had adenocarcinoma, 4% had large cell, and 28% had other histologies.
Efficacy results are presented in Table 10 and Figure 4.
| Efficacy Parameter | Pemetrexed | Placebo |
|---|---|---|
| ||
| Overall survival | N=441 | N=222 |
| Median (months) | 13.4 | 10.6 |
| (95% CI) | (11.9–15.9) | (8.7–12.0) |
| Hazard ratio* | 0.79 | |
| (95% CI) | (0.65–0.95) | |
| p-value | p=0.012 | |
| Progression-free survival per independent review | N=387 | N=194 |
| Median (months) | 4.0 | 2.0 |
| (95% CI) | (3.1–4.4) | (1.5–2.8) |
| Hazard ratio* | 0.60 | |
| (95% CI) | (0.49–0.73) | |
| p-value | p<0.00001 | |

Figure 4: Kaplan-Meier Curves for Overall Survival in Study JMEN
The results of pre-specified subgroup analyses by NSCLC histology are presented in Table 11 and Figures 5 and 6.
| Efficacy Parameter | Overall Survival | Progression-Free Survival Per Independent Review | ||
|---|---|---|---|---|
| Pemetrexed (N=441) | Placebo (N=222) | Pemetrexed (N=387) | Placebo (N=194) | |
| Non-squamous NSCLC (n=481) | ||||
| Median (months) | 15.5 | 10.3 | 4.4 | 1.8 |
| HR* | 0.70 | 0.47 | ||
| (95% CI) | (0.56–0.88) | (0.37–0.60) | ||
| Adenocarcinoma (n=328) | ||||
| Median (months) | 16.8 | 11.5 | 4.6 | 2.7 |
| HR* | 0.73 | 0.51 | ||
| (95% CI) | (0.56–0.96) | (0.38–0.68) | ||
| Large cell carcinoma (n=20) | ||||
| Median (months) | 8.4 | 7.9 | 4.5 | 1.5 |
| HR* | 0.98 | 0.40 | ||
| (95% CI) | (0.36–2.65) | (0.12–1.29) | ||
| Other† (n=133) | ||||
| Median (months) | 11.3 | 7.7 | 4.1 | 1.6 |
| HR* | 0.61 | 0.44 | ||
| (95% CI) | (0.40–0.94) | (0.28–0.68) | ||
| Squamous cell NSCLC (n=182) | ||||
| Median (months) | 9.9 | 10.8 | 2.4 | 2.5 |
| HR* | 1.07 | 1.03 | ||
| (95% CI) | (0.77–1.50) | (0.71–1.49) | ||

Figure 5: Kaplan-Meier Curves for Overall Survival in Non-squamous NSCLC in Study JMEN

Figure 6: Kaplan-Meier Curves for Overall Survival in Squamous NSCLC in Study JMEN
Maintenance Treatment Following First-line Pemetrexed Plus Platinum Chemotherapy
The efficacy of pemetrexed as maintenance therapy following first-line platinum-based chemotherapy was also evaluated in PARAMOUNT (NCT00789373), a multi-center, randomized (2:1), double-blind, placebo-controlled study conducted in patients with Stage IIIb/IV non-squamous NSCLC who had completed four cycles of pemetrexed in combination with cisplatin and achieved a complete response (CR) or partial response (PR) or stable disease (SD). Patients were required to have an ECOG PS of 0 or 1. Patients were randomized to receive pemetrexed 500 mg/m2 intravenously every 21 days or placebo until disease progression. Randomization was stratified by response to pemetrexed in combination with cisplatin induction therapy (CR or PR versus SD), disease stage (IIIb versus IV), and ECOG PS (0 versus 1). Patients in both arms received folic acid, vitamin B12, and dexamethasone. The main efficacy outcome measure was investigator-assessed progression-free survival (PFS) and an additional efficacy outcome measure was overall survival (OS); PFS and OS were measured from the time of randomization.
A total of 539 patients were enrolled with 359 patients randomized to pemetrexed and 180 patients randomized to placebo. The median age was 61 years (range 32 to 83 years); 58% were male; 95% were White, 4.5% were Asian, and <1% were Black or African American; 67% had an ECOG PS of 1; 78% were current or former smokers; and 43% of the population achieved a partial or complete response to first-line, platinum-based chemotherapy. With regard to tumor characteristics, 91% had Stage IV disease, 87% had adenocarcinoma, 7% had large cell, and 6% had other histologies.
Efficacy results for PARAMOUNT are presented in Table 12 and Figure 7.
| Efficacy Parameter | Pemetrexed (N=359) | Placebo (N=180) |
|---|---|---|
| Overall survival | ||
| Median (months) | 13.9 | 11.0 |
| (95% CI) | (12.8–16.0) | (10.0–12.5) |
| Hazard ratio (HR)* | 0.78 | |
| (95% CI) | (0.64–0.96) | |
| p-value | p=0.02 | |
| Progression-free survival† | ||
| Median (months) | 4.1 | 2.8 |
| (95% CI) | (3.2–4.6) | (2.6–3.1) |
| Hazard ratio (HR)* | 0.62 | |
| (95% CI) | (0.49–0.79) | |
| p-value | p<0.0001 | |

Figure 7: Kaplan-Meier Curves for Overall Survival in PARAMOUNT
Treatment of Recurrent Disease After Prior Chemotherapy
The efficacy of pemetrexed was evaluated in Study JMEI (NCT00004881), a multicenter, randomized (1:1), open-label study conducted in patients with Stage III or IV NSCLC that had recurred or progressed following one prior chemotherapy regimen for advanced disease. Patients were randomized to receive pemetrexed 500 mg/m2 intravenously or docetaxel 75 mg/m2 as a 1-hour intravenous infusion once every 21 days. Patients randomized to pemetrexed also received folic acid and vitamin B12. The study was designed to show that overall survival with pemetrexed was non-inferior to docetaxel, as the major efficacy outcome measure, and that overall survival was superior for patients randomized to pemetrexed compared to docetaxel, as a secondary outcome measure.
A total of 571 patients were enrolled with 283 patients randomized to pemetrexed and 288 patients randomized to docetaxel. The median age was 58 years (range 22 to 87 years); 72% were male; 71% were White, 24% were Asian, 2.8% were Black or African American, 1.8% were Hispanic or Latino, and <2% were other ethnicities; 88% had an ECOG PS of 0 or 1. With regard to tumor characteristics, 75% had Stage IV disease; 53% had adenocarcinoma, 30% had squamous histology; 8% large cell; and 9% had other histologic subtypes of NSCLC.
The efficacy results in the overall population and in subgroup analyses based on histologic subtype are provided in Tables 13 and 14, respectively. Study JMEI did not show an improvement in overall survival in the intent-to-treat population. In subgroup analyses, there was no evidence of a treatment effect on survival in patients with squamous NSCLC; the absence of a treatment effect in patients with NSCLC of squamous histology was also observed in Studies JMDB and JMEN [see Clinical Studies (14.1)].
| Efficacy Parameter | Pemetrexed (N=283) | Docetaxel (N=288) |
|---|---|---|
| ||
| Overall survival | ||
| Median (months) | 8.3 | 7.9 |
| (95% CI) | (7.0–9.4) | (6.3–9.2) |
| Hazard ratio* | 0.99 | |
| (95% CI) | (0.82–1.20) | |
| Progression-free survival | ||
| Median (months) | 2.9 | 2.9 |
| (95% CI) | (2.4–3.1) | (2.7–3.4) |
| Hazard ratio* | 0.97 | |
| (95% CI) | (0.82–1.16) | |
| Overall response rate | 8.5% | 8.3% |
| (95% CI) | (5.2–11.7) | (5.1–11.5) |
| Histologic Subgroups | Pemetrexed (N=283) | Docetaxel (N=288) |
|---|---|---|
| Non-squamous NSCLC (N=399) | ||
| Median (months) | 9.3 | 8.0 |
| (95% CI) | (7.8–9.7) | (6.3–9.3) |
| HR* | 0.89 | |
| (95% CI) | (0.71–1.13) | |
| Adenocarcinoma (N=301) | ||
| Median (months) | 9.0 | 9.2 |
| (95% CI) | (7.6–9.6) | (7.5–11.3) |
| HR* | 1.09 | |
| (95% CI) | (0.83–1.44) | |
| Large Cell (N=47) | ||
| Median (months) | 12.8 | 4.5 |
| (95% CI) | (5.8–14.0) | (2.3–9.1) |
| HR* | 0.38 | |
| (95% CI) | (0.18–0.78) | |
| Other† (N=51) | ||
| Median (months) | 9.4 | 7.9 |
| (95% CI) | (6.0–10.1) | (4.0–8.9) |
| HR* | 0.62 | |
| (95% CI) | (0.32–1.23) | |
| Squamous NSCLC (N=172) | ||
| Median (months) | 6.2 | 7.4 |
| (95% CI) | (4.9–8.0) | (5.6–9.5) |
| HR* | 1.32 | |
| (95% CI) | (0.93–1.86) | |
14.2 Mesothelioma
The efficacy of pemetrexed was evaluated in Study JMCH (NCT00005636), a multicenter, randomized (1:1), single-blind study conducted in patients with MPM who had received no prior chemotherapy. Patients were randomized (n=456) to receive pemetrexed 500 mg/m2 intravenously over 10 minutes followed 30 minutes later by cisplatin 75 mg/m2 intravenously over two hours on Day 1 of each 21-day cycle or to receive cisplatin 75 mg/m2 intravenously over 2 hours on Day 1 of each 21-day cycle; treatment continued until disease progression or intolerable toxicity. The study was modified after randomization and treatment of 117 patients to require that all patients receive folic acid 350 mcg to 1000 mcg daily beginning 1 to 3 weeks prior to the first dose of pemetrexed and continuing until 1 to 3 weeks after the last dose, vitamin B12 1000 mcg intramuscularly 1 to 3 weeks prior to first dose of pemetrexed and every 9 weeks thereafter, and dexamethasone 4 mg orally, twice daily, for 3 days starting the day prior to each pemetrexed dose. Randomization was stratified by multiple baseline variables including KPS, histologic subtype (epithelial, mixed, sarcomatoid, other), and gender. The major efficacy outcome measure was overall survival and additional efficacy outcome measures were time to disease progression, overall response rate, and response duration.
A total of 448 patients received at least one dose of protocol-specified therapy; 226 patients were randomized to and received at least one dose of pemetrexed plus cisplatin, and 222 patients were randomized to and received cisplatin. Among the 226 patients who received cisplatin with pemetrexed, 74% received full supplementation with folic acid and vitamin B12 during study therapy, 14% were never supplemented, and 12% were partially supplemented. Across the study population, the median age was 61 years (range: 20 to 86 years); 81% were male; 92% were White, 5% were Hispanic or Latino, 3.1% were Asian, and <1% were other ethnicities; and 54% had a baseline KPS score of 90–100% and 46% had a KPS score of 70–80%. With regard to tumor characteristics, 46% had Stage IV disease, 31% Stage III, 15% Stage II, and 7% Stage I disease at baseline; the histologic subtype of mesothelioma was epithelial in 68% of patients, mixed in 16%, sarcomatoid in 10% and other histologic subtypes in 6%. The baseline demographics and tumor characteristics of the subgroup of fully supplemented patients was similar to the overall study population.
The efficacy results from Study JMCH are summarized in Table 15 and Figure 8.
| Efficacy Parameter | All Randomized and Treated Patients (N=448) | Fully Supplemented Patients (N=331) | ||
|---|---|---|---|---|
| Pemetrexed/Cisplatin (N=226) | Cisplatin (N=222) | Pemetrexed/Cisplatin (N=168) | Cisplatin (N=163) | |
| Median overall survival (months) | 12.1 | 9.3 | 13.3 | 10.0 |
| (95% CI) | (10.0–14.4) | (7.8–10.7) | (11.4–14.9) | (8.4–11.9) |
| Hazard ratio* | 0.77 | 0.75 | ||
| Log rank p-value | 0.020 | NA† | ||

Figure 8: Kaplan-Meier Curves for Overall Survival in Study JMCH
Based upon prospectively defined criteria (modified Southwest Oncology Group methodology) the objective tumor response rate for pemetrexed plus cisplatin was greater than the objective tumor response rate for cisplatin alone. There was also improvement in lung function (forced vital capacity) in the pemetrexed plus cisplatin arm compared to the control arm.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.