XALKORI® Full Patient Information
(crizotinib)
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide; Instructions for Use).
Hepatotoxicity
Inform patients to immediately report symptoms of hepatotoxicity [see Warnings and Precautions (5.1)].
Interstitial Lung Disease (Pneumonitis)
Advise patients to immediately report any new or worsening pulmonary symptoms [see Warnings and Precautions (5.2)].
Bradycardia
Advise patients to report any symptoms of bradycardia and to inform their healthcare provider about the use of any heart or blood pressure medications [see Warnings and Precautions (5.4)].
Severe Visual Loss
Inform patients of the potential risk of severe visual loss and to immediately contact their healthcare provider if they develop severe visual loss. Inform patients that visual changes such as perceived flashes of light, blurry vision, light sensitivity, and floaters are commonly reported adverse reactions and may occur while driving or operating machinery. The onset of visual disorders most commonly occurs during the first week of treatment [see Warnings and Precautions (5.5), Adverse Reactions (6)].
Gastrointestinal Toxicity in Pediatric and Young Adult Patients with ALCL or Pediatric Patients with IMT
Inform patients with ALCL or pediatric patients with IMT of the risk of severe nausea, vomiting, diarrhea, and stomatitis. Advise patients to immediately inform their healthcare provider of problems with swallowing, vomiting, or diarrhea [see Warnings and Precautions (5.6)].
Drug Interactions
Inform patients to avoid grapefruit or grapefruit juice while taking XALKORI. Advise patients to inform their healthcare providers of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products [see Drug Interactions (7)].
Photosensitivity
Inform patients of the signs and symptoms of photosensitivity. Advise patients to avoid prolonged sun exposure and to use sunscreen or protective clothing during treatment with XALKORI [see Adverse Reactions (6.1)].
Dosage and Administration
Advise patients to take XALKORI with or without food. If a patient misses a dose, advise the patient to take it as soon as remembered unless it is less than 6 hours until the next dose, in which case, advise the patient not to take the missed dose. If a patient vomits after taking a dose of XALKORI, advise the patient not to take an extra dose, but to take the next dose at the regular time [see Dosage and Administration (2.4)].
Capsules:
Advise patients to swallow XALKORI capsules whole [see Dosage and Administration (2.4)].
Oral Pellets:
Inform patient or caregiver to open the encapsulated XALKORI oral pellets and administer the oral pellets directly in the patient’s mouth or with a consumer-supplied oral dosing aid, for example a spoon or medicine cup. Advise patient or caregiver that the oral pellets are not to be chewed and to give a sufficient amount of water after pellets are administered to ensure all oral pellets are swallowed [see Dosage and Administration (2.4)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.7), Use in Specific Populations (8.1)].
Females and Males of Reproductive Potential
Advise females of reproductive potential to use effective contraception during treatment with XALKORI and for 45 days after the last dose [see Use in Specific Populations (8.3)].
Advise male patients with female partners of reproductive potential to use condoms during treatment with XALKORI and for 90 days after the last dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise females not to breastfeed during treatment with XALKORI and for 45 days after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise females and males of reproductive potential of the potential for reduced fertility from XALKORI [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: Sep 2023 | |||
MEDICATION GUIDE | ||||
XALKORI (zal-KOR-ee) | XALKORI (zal-KOR-ee) | |||
What is the most important information I should know about XALKORI?
| ||||
|
| |||
| ||||
|
| |||
In addition, for children or young adults taking XALKORI to treat anaplastic large cell lymphoma (ALCL) or children taking XALKORI to treat inflammatory myofibroblastic tumor (IMT): | ||||
See "What are possible side effects of XALKORI?" for more information about side effects. | ||||
What is XALKORI?
It is not known if XALKORI is safe and effective in children younger than 1 year of age with ALCL or IMT, or in any children with NSCLC. | ||||
Before taking XALKORI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. XALKORI can affect the way other medicines work, and other medicines can affect how XALKORI works. | ||||
How should I take XALKORI?
XALKORI comes as capsules and oral pellets.
| ||||
What should I avoid while taking XALKORI?
| ||||
What are the possible side effects of XALKORI? The most common side effects of XALKORI in adults with NSCLC include: | ||||
|
| |||
The most common side effects of XALKORI in people with ALCL include: | ||||
|
| |||
The most common side effects of XALKORI in adults with IMT include: | ||||
| ||||
The most common side effects of XALKORI in children with IMT include: | ||||
|
| |||
XALKORI may cause fertility problems in females and males, which may affect the ability to have children. Talk to your healthcare provider if you have concerns about fertility. | ||||
How should I store XALKORI?
Keep XALKORI and all medicines out of the reach of children. | ||||
General information about the safe and effective use of XALKORI. | ||||
What are the ingredients in XALKORI?  LAB-0441-12.0 | ||||
Instructions for Use
INSTRUCTIONS FOR USE
XALKORI [zal-KOR-ee]
(crizotinib)
oral pellets
This Instructions for Use contains information on how to give or take XALKORI oral pellets. Read this Instructions for Use each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider or pharmacist about your or your child’s medical condition or treatment.
Important information you need to know before giving or taking XALKORI oral pellets:
- •
- XALKORI oral pellets come in a capsule “shell” that must be opened before giving or taking a dose. Do not swallow the shell containing the oral pellets. Do not chew or crush the oral pellets.
- •
- XALKORI oral pellets come in 3 dosage strengths: 20 mg, 50 mg, and 150 mg. Your healthcare provider may combine different strengths for your prescribed dose. No more than 4 XALKORI oral pellet shells are to be used for a single dose.
- •
- Your healthcare provider will decide the right dose of XALKORI oral pellets for you or your child. Follow your healthcare provider’s instructions for the dose of XALKORI oral pellets to give your child or for you to take.
- •
- Empty XALKORI oral pellets from the shells as described in Steps 1 to 4 below.
- •
- Check the expiration date on the bottle containing XALKORI oral pellets. Do not use XALKORI oral pellets after the expiration date on the bottle has passed.
- •
- Ask your healthcare provider or pharmacist if you are not sure how to prepare and give or take the prescribed dose of XALKORI oral pellets.
Supplies needed to give or take XALKORI oral pellets:
- •
- XALKORI oral pellet(s), as prescribed by your healthcare provider.
- •
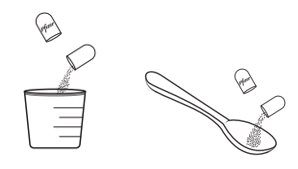
- Spoon or medicine cup (optional). See Step 4 “Giving or taking XALKORI oral pellets”.
Preparing XALKORI oral pellets (Steps 1 to 3):
Giving or taking XALKORI oral pellets (Step 4): There are 2 options for giving or taking the oral pellets.
Storing XALKORI oral pellets:
- •
- Store XALKORI oral pellets at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Keep XALKORI oral pellets and all medicines out of the reach of children.
Disposing of empty XALKORI oral pellet shells:
- •
- Dispose of (throw away) the empty XALKORI oral pellet shell(s) in the household trash.
- •
- Ask your pharmacist how to throw away medicines you no longer use or are expired.
For more information, go to www.Pfizer.com or call 1-800-438-1985.
LAB-1526-1.0
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 09/2023
Find XALKORI® medical information:
Find XALKORI® medical information:
XALKORI® Quick Finder
Health Professional Information
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide; Instructions for Use).
Hepatotoxicity
Inform patients to immediately report symptoms of hepatotoxicity [see Warnings and Precautions (5.1)].
Interstitial Lung Disease (Pneumonitis)
Advise patients to immediately report any new or worsening pulmonary symptoms [see Warnings and Precautions (5.2)].
Bradycardia
Advise patients to report any symptoms of bradycardia and to inform their healthcare provider about the use of any heart or blood pressure medications [see Warnings and Precautions (5.4)].
Severe Visual Loss
Inform patients of the potential risk of severe visual loss and to immediately contact their healthcare provider if they develop severe visual loss. Inform patients that visual changes such as perceived flashes of light, blurry vision, light sensitivity, and floaters are commonly reported adverse reactions and may occur while driving or operating machinery. The onset of visual disorders most commonly occurs during the first week of treatment [see Warnings and Precautions (5.5), Adverse Reactions (6)].
Gastrointestinal Toxicity in Pediatric and Young Adult Patients with ALCL or Pediatric Patients with IMT
Inform patients with ALCL or pediatric patients with IMT of the risk of severe nausea, vomiting, diarrhea, and stomatitis. Advise patients to immediately inform their healthcare provider of problems with swallowing, vomiting, or diarrhea [see Warnings and Precautions (5.6)].
Drug Interactions
Inform patients to avoid grapefruit or grapefruit juice while taking XALKORI. Advise patients to inform their healthcare providers of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products [see Drug Interactions (7)].
Photosensitivity
Inform patients of the signs and symptoms of photosensitivity. Advise patients to avoid prolonged sun exposure and to use sunscreen or protective clothing during treatment with XALKORI [see Adverse Reactions (6.1)].
Dosage and Administration
Advise patients to take XALKORI with or without food. If a patient misses a dose, advise the patient to take it as soon as remembered unless it is less than 6 hours until the next dose, in which case, advise the patient not to take the missed dose. If a patient vomits after taking a dose of XALKORI, advise the patient not to take an extra dose, but to take the next dose at the regular time [see Dosage and Administration (2.4)].
Capsules:
Advise patients to swallow XALKORI capsules whole [see Dosage and Administration (2.4)].
Oral Pellets:
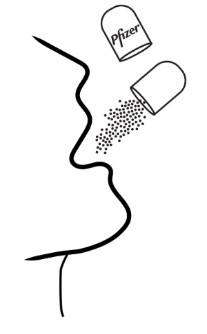
Inform patient or caregiver to open the encapsulated XALKORI oral pellets and administer the oral pellets directly in the patient’s mouth or with a consumer-supplied oral dosing aid, for example a spoon or medicine cup. Advise patient or caregiver that the oral pellets are not to be chewed and to give a sufficient amount of water after pellets are administered to ensure all oral pellets are swallowed [see Dosage and Administration (2.4)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.7), Use in Specific Populations (8.1)].
Females and Males of Reproductive Potential
Advise females of reproductive potential to use effective contraception during treatment with XALKORI and for 45 days after the last dose [see Use in Specific Populations (8.3)].
Advise male patients with female partners of reproductive potential to use condoms during treatment with XALKORI and for 90 days after the last dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise females not to breastfeed during treatment with XALKORI and for 45 days after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise females and males of reproductive potential of the potential for reduced fertility from XALKORI [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: Sep 2023 | |||
MEDICATION GUIDE | ||||
XALKORI (zal-KOR-ee) | XALKORI (zal-KOR-ee) | |||
What is the most important information I should know about XALKORI?
| ||||
|
| |||
| ||||
|
| |||
In addition, for children or young adults taking XALKORI to treat anaplastic large cell lymphoma (ALCL) or children taking XALKORI to treat inflammatory myofibroblastic tumor (IMT): | ||||
See "What are possible side effects of XALKORI?" for more information about side effects. | ||||
What is XALKORI?
It is not known if XALKORI is safe and effective in children younger than 1 year of age with ALCL or IMT, or in any children with NSCLC. | ||||
Before taking XALKORI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. XALKORI can affect the way other medicines work, and other medicines can affect how XALKORI works. | ||||
How should I take XALKORI?
XALKORI comes as capsules and oral pellets.
| ||||
What should I avoid while taking XALKORI?
| ||||
What are the possible side effects of XALKORI? The most common side effects of XALKORI in adults with NSCLC include: | ||||
|
| |||
The most common side effects of XALKORI in people with ALCL include: | ||||
|
| |||
The most common side effects of XALKORI in adults with IMT include: | ||||
| ||||
The most common side effects of XALKORI in children with IMT include: | ||||
|
| |||
XALKORI may cause fertility problems in females and males, which may affect the ability to have children. Talk to your healthcare provider if you have concerns about fertility. | ||||
How should I store XALKORI?
Keep XALKORI and all medicines out of the reach of children. | ||||
General information about the safe and effective use of XALKORI. | ||||
What are the ingredients in XALKORI?  LAB-0441-12.0 | ||||
Instructions for Use
INSTRUCTIONS FOR USE
XALKORI [zal-KOR-ee]
(crizotinib)
oral pellets
This Instructions for Use contains information on how to give or take XALKORI oral pellets. Read this Instructions for Use each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider or pharmacist about your or your child’s medical condition or treatment.
Important information you need to know before giving or taking XALKORI oral pellets:
- •
- XALKORI oral pellets come in a capsule “shell” that must be opened before giving or taking a dose. Do not swallow the shell containing the oral pellets. Do not chew or crush the oral pellets.
- •
- XALKORI oral pellets come in 3 dosage strengths: 20 mg, 50 mg, and 150 mg. Your healthcare provider may combine different strengths for your prescribed dose. No more than 4 XALKORI oral pellet shells are to be used for a single dose.
- •
- Your healthcare provider will decide the right dose of XALKORI oral pellets for you or your child. Follow your healthcare provider’s instructions for the dose of XALKORI oral pellets to give your child or for you to take.
- •
- Empty XALKORI oral pellets from the shells as described in Steps 1 to 4 below.
- •
- Check the expiration date on the bottle containing XALKORI oral pellets. Do not use XALKORI oral pellets after the expiration date on the bottle has passed.
- •
- Ask your healthcare provider or pharmacist if you are not sure how to prepare and give or take the prescribed dose of XALKORI oral pellets.
Supplies needed to give or take XALKORI oral pellets:
- •
- XALKORI oral pellet(s), as prescribed by your healthcare provider.
- •
- Spoon or medicine cup (optional). See Step 4 “Giving or taking XALKORI oral pellets”.
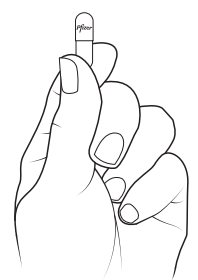
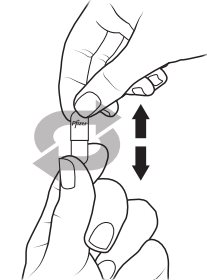
Preparing XALKORI oral pellets (Steps 1 to 3):
Giving or taking XALKORI oral pellets (Step 4): There are 2 options for giving or taking the oral pellets.
Storing XALKORI oral pellets:
- •
- Store XALKORI oral pellets at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Keep XALKORI oral pellets and all medicines out of the reach of children.
Disposing of empty XALKORI oral pellet shells:
- •
- Dispose of (throw away) the empty XALKORI oral pellet shell(s) in the household trash.
- •
- Ask your pharmacist how to throw away medicines you no longer use or are expired.
For more information, go to www.Pfizer.com or call 1-800-438-1985.
LAB-1526-1.0
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 09/2023
Health Professional Information
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide; Instructions for Use).
Hepatotoxicity
Inform patients to immediately report symptoms of hepatotoxicity [see Warnings and Precautions (5.1)].
Interstitial Lung Disease (Pneumonitis)
Advise patients to immediately report any new or worsening pulmonary symptoms [see Warnings and Precautions (5.2)].
Bradycardia
Advise patients to report any symptoms of bradycardia and to inform their healthcare provider about the use of any heart or blood pressure medications [see Warnings and Precautions (5.4)].
Severe Visual Loss
Inform patients of the potential risk of severe visual loss and to immediately contact their healthcare provider if they develop severe visual loss. Inform patients that visual changes such as perceived flashes of light, blurry vision, light sensitivity, and floaters are commonly reported adverse reactions and may occur while driving or operating machinery. The onset of visual disorders most commonly occurs during the first week of treatment [see Warnings and Precautions (5.5), Adverse Reactions (6)].
Gastrointestinal Toxicity in Pediatric and Young Adult Patients with ALCL or Pediatric Patients with IMT
Inform patients with ALCL or pediatric patients with IMT of the risk of severe nausea, vomiting, diarrhea, and stomatitis. Advise patients to immediately inform their healthcare provider of problems with swallowing, vomiting, or diarrhea [see Warnings and Precautions (5.6)].
Drug Interactions
Inform patients to avoid grapefruit or grapefruit juice while taking XALKORI. Advise patients to inform their healthcare providers of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products [see Drug Interactions (7)].
Photosensitivity
Inform patients of the signs and symptoms of photosensitivity. Advise patients to avoid prolonged sun exposure and to use sunscreen or protective clothing during treatment with XALKORI [see Adverse Reactions (6.1)].
Dosage and Administration
Advise patients to take XALKORI with or without food. If a patient misses a dose, advise the patient to take it as soon as remembered unless it is less than 6 hours until the next dose, in which case, advise the patient not to take the missed dose. If a patient vomits after taking a dose of XALKORI, advise the patient not to take an extra dose, but to take the next dose at the regular time [see Dosage and Administration (2.4)].
Capsules:
Advise patients to swallow XALKORI capsules whole [see Dosage and Administration (2.4)].
Oral Pellets:
Inform patient or caregiver to open the encapsulated XALKORI oral pellets and administer the oral pellets directly in the patient’s mouth or with a consumer-supplied oral dosing aid, for example a spoon or medicine cup. Advise patient or caregiver that the oral pellets are not to be chewed and to give a sufficient amount of water after pellets are administered to ensure all oral pellets are swallowed [see Dosage and Administration (2.4)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.7), Use in Specific Populations (8.1)].
Females and Males of Reproductive Potential
Advise females of reproductive potential to use effective contraception during treatment with XALKORI and for 45 days after the last dose [see Use in Specific Populations (8.3)].
Advise male patients with female partners of reproductive potential to use condoms during treatment with XALKORI and for 90 days after the last dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise females not to breastfeed during treatment with XALKORI and for 45 days after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise females and males of reproductive potential of the potential for reduced fertility from XALKORI [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: Sep 2023 | |||
MEDICATION GUIDE | ||||
XALKORI (zal-KOR-ee) | XALKORI (zal-KOR-ee) | |||
What is the most important information I should know about XALKORI?
| ||||
|
| |||
| ||||
|
| |||
In addition, for children or young adults taking XALKORI to treat anaplastic large cell lymphoma (ALCL) or children taking XALKORI to treat inflammatory myofibroblastic tumor (IMT): | ||||
See "What are possible side effects of XALKORI?" for more information about side effects. | ||||
What is XALKORI?
It is not known if XALKORI is safe and effective in children younger than 1 year of age with ALCL or IMT, or in any children with NSCLC. | ||||
Before taking XALKORI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. XALKORI can affect the way other medicines work, and other medicines can affect how XALKORI works. | ||||
How should I take XALKORI?
XALKORI comes as capsules and oral pellets.
| ||||
What should I avoid while taking XALKORI?
| ||||
What are the possible side effects of XALKORI? The most common side effects of XALKORI in adults with NSCLC include: | ||||
|
| |||
The most common side effects of XALKORI in people with ALCL include: | ||||
|
| |||
The most common side effects of XALKORI in adults with IMT include: | ||||
| ||||
The most common side effects of XALKORI in children with IMT include: | ||||
|
| |||
XALKORI may cause fertility problems in females and males, which may affect the ability to have children. Talk to your healthcare provider if you have concerns about fertility. | ||||
How should I store XALKORI?
Keep XALKORI and all medicines out of the reach of children. | ||||
General information about the safe and effective use of XALKORI. | ||||
What are the ingredients in XALKORI?  LAB-0441-12.0 | ||||
Instructions for Use
INSTRUCTIONS FOR USE
XALKORI [zal-KOR-ee]
(crizotinib)
oral pellets
This Instructions for Use contains information on how to give or take XALKORI oral pellets. Read this Instructions for Use each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider or pharmacist about your or your child’s medical condition or treatment.
Important information you need to know before giving or taking XALKORI oral pellets:
- •
- XALKORI oral pellets come in a capsule “shell” that must be opened before giving or taking a dose. Do not swallow the shell containing the oral pellets. Do not chew or crush the oral pellets.
- •
- XALKORI oral pellets come in 3 dosage strengths: 20 mg, 50 mg, and 150 mg. Your healthcare provider may combine different strengths for your prescribed dose. No more than 4 XALKORI oral pellet shells are to be used for a single dose.
- •
- Your healthcare provider will decide the right dose of XALKORI oral pellets for you or your child. Follow your healthcare provider’s instructions for the dose of XALKORI oral pellets to give your child or for you to take.
- •
- Empty XALKORI oral pellets from the shells as described in Steps 1 to 4 below.
- •
- Check the expiration date on the bottle containing XALKORI oral pellets. Do not use XALKORI oral pellets after the expiration date on the bottle has passed.
- •
- Ask your healthcare provider or pharmacist if you are not sure how to prepare and give or take the prescribed dose of XALKORI oral pellets.
Supplies needed to give or take XALKORI oral pellets:
- •
- XALKORI oral pellet(s), as prescribed by your healthcare provider.
- •
- Spoon or medicine cup (optional). See Step 4 “Giving or taking XALKORI oral pellets”.
Preparing XALKORI oral pellets (Steps 1 to 3):
Giving or taking XALKORI oral pellets (Step 4): There are 2 options for giving or taking the oral pellets.
Storing XALKORI oral pellets:
- •
- Store XALKORI oral pellets at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Keep XALKORI oral pellets and all medicines out of the reach of children.
Disposing of empty XALKORI oral pellet shells:
- •
- Dispose of (throw away) the empty XALKORI oral pellet shell(s) in the household trash.
- •
- Ask your pharmacist how to throw away medicines you no longer use or are expired.
For more information, go to www.Pfizer.com or call 1-800-438-1985.
LAB-1526-1.0
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 09/2023
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information.9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.