RETACRIT™ Full Patient Information
(epoetin alfa-epbx)
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Inform patients:
- •
- Of the increased risks of mortality, serious cardiovascular reactions, thromboembolic reactions, stroke, and tumor progression [see Warnings and Precautions (5.1, 5.2)].
- •
- To undergo regular blood pressure monitoring, adhere to prescribed anti-hypertensive regimen and follow recommended dietary restrictions.
- •
- To contact their healthcare provider for new-onset neurologic symptoms or change in seizure frequency.
- •
- Of the need to have regular laboratory tests for hemoglobin.
- •
- Risks are associated with benzyl alcohol in neonates, infants, pregnant women, and lactating women [see Use in Specific Populations (8.1, 8.2, 8.4)].
Instruct patients who self-administer RETACRIT of the:
- •
- Importance of following the Instructions for Use.
- •
- Dangers of reusing needles, syringes, or unused portions of single-dose vials.
- •
- Proper disposal of used syringes, needles, and unused vials, and of the full container.
| This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 6/2024 | ||
MEDICATION GUIDE | ||
Read this Medication Guide:
This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment. Talk with your healthcare provider regularly about the use of RETACRIT and ask if there is new information about RETACRIT. | ||
What is the most important information I should know about RETACRIT? RETACRIT may cause serious side effects that can lead to death, including: For people with cancer:
For all people who take RETACRIT, including people with cancer or chronic kidney disease:
See "What are the possible side effects of RETACRIT?" below for more information. If you decide to take RETACRIT, your healthcare provider should prescribe the smallest dose of RETACRIT that is necessary to reduce your chance of needing RBC transfusions. | ||
What is RETACRIT? RETACRIT is a prescription medicine used to treat anemia. People with anemia have a lower-than-normal number of RBCs. RETACRIT works like the human protein called erythropoietin to help your body make more RBCs. RETACRIT is used to reduce or avoid the need for RBC transfusions. RETACRIT may be used to treat anemia if it is caused by:
RETACRIT may also be used to reduce the chance you will need RBC transfusions if you are scheduled for certain surgeries where a lot of blood loss is expected. If your hemoglobin level stays too high or if your hemoglobin goes up too quickly, this may lead to serious health problems which may result in death. These serious health problems may happen if you take RETACRIT, even if you do not have an increase in your hemoglobin level. RETACRIT has not been proven to improve quality of life, fatigue, or well-being. RETACRIT should not be used for treatment of anemia:
RETACRIT should not be used to reduce the chance you will need RBC transfusions if:
It is not known if RETACRIT is safe and effective in treating anemia in children less than 1 month old who have chronic kidney disease and in children less than 5 years old who have anemia caused by chemotherapy. | ||
Do not take RETACRIT if you:
Do not give RETACRIT from multiple-dose vials to:
| ||
Before taking RETACRIT, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | ||
How should I take RETACRIT?
| ||
What are the possible side effects of RETACRIT? RETACRIT may cause serious side effects, including:
Common side effects of RETACRIT include: | ||
|
|
|
These are not all of the possible side effects of RETACRIT. Your healthcare provider can give you a more complete list. Tell your healthcare provider about any side effects that bother you or that do not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | ||
How should I store RETACRIT?
Keep RETACRIT and all medicines out of the reach of children. | ||
General information about the safe and effective use of RETACRIT. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use RETACRIT for a condition for which it was not prescribed. Do not give RETACRIT to other people even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about RETACRIT that is written for healthcare professionals. | ||
What are the ingredients in RETACRIT? Active Ingredient: epoetin alfa-epbx Inactive Ingredients:
Manufactured by Hospira, Inc., a Pfizer Company, Lake Forest, IL 60045 USA  LAB-0827-9.0 | ||
Instructions for Use
RETACRIT (Ret-uh-krit)
(epoetin alfa-epbx)
Use these Instructions for Use if you or your caregiver has been trained to give RETACRIT injections at home. Do not give yourself the injection unless you have received training from your healthcare provider. If you are not sure about giving the injection or you have questions, ask your healthcare provider for help.
Before reading these Instructions for Use, read the Medication Guide that comes with RETACRIT for the most important information you need to know.
When you receive your RETACRIT vial make sure that:
- •
- The name RETACRIT appears on the carton and vial label.
- •
- The expiration date on the vial label has not passed. Do not use a vial of RETACRIT after the expiration date on the label.
- •
- The dose strength of the RETACRIT vial (number of Units per mL on the vial label) is the same as your healthcare provider prescribed.
- •
- You understand what the dose strength of RETACRIT means. RETACRIT vials come in several dose strengths. For example, the dose strength may be described as 10,000 Units/mL on the vial label. This strength means that 10,000 Units of medicine are contained in each 1 mL (milliliter) of liquid. Your healthcare provider may also refer to a mL as a "cc." One mL is the same as one "cc."
- •
- The RETACRIT liquid in the vial is clear and colorless. Do not use RETACRIT if the liquid in the vial looks discolored or cloudy, or if the liquid has lumps, flakes, or particles.
- •
- The RETACRIT vial has a color cap on the top of the vial. Do not use a vial of RETACRIT if the color cap on the top of the vial has been removed or is missing.
- •
- Use only the type of disposable syringe and needle that your healthcare provider has prescribed.
- •
- Do not shake RETACRIT. Shaking could cause RETACRIT not to work. If you shake RETACRIT, the solution in the vial may look foamy and should not be used.
- •
- Do not freeze RETACRIT. Do not use a vial of RETACRIT that has been frozen.
- •
- Do not use the carton of RETACRIT multiple-dose vials if it has been frozen or if the green area on the freeze strip indicator inside the RETACRIT carton looks white or cloudy.
- •
- Store RETACRIT in the refrigerator between 36°F to 46°F (2°C to 8°C).
- •
- Keep RETACRIT away from light.
- •
- Single-dose vials of RETACRIT should be used only one time. Throw the vial away after use even if there is medicine left in the vial.
- •
- After removing a dose from the multiple-dose vial, store the vial in the refrigerator (but not the freezer). Do not store the vial for more than 21 days.
- •
- Throw away the multiple-dose vials as directed by your healthcare provider:
- o
- if there is not enough medicine left in the multiple-dose vial for another dose, or
- o
- if it has been more than 21 days since you first put a needle into the multiple-dose vial.
How should I prepare for an injection of RETACRIT?
- •
- Always keep an extra syringe and needle on hand.
- •
- Follow your healthcare provider's instructions on how to measure your dose of RETACRIT. This dose will be measured in Units per mL or cc (1 mL is the same as 1 cc). Use a syringe that is marked in tenths of mL (for example, 0.2 mL or 0.2 cc). Using the wrong syringe can lead to a mistake in your dose and you could inject too much or too little RETACRIT.
Only use disposable syringes and needles. Use the syringes and needles only one time and then throw them away as instructed by your healthcare provider.
What do I need to know about the different types of RETACRIT vials?
RETACRIT comes in two different types of vials:
- •
- Single-dose vials
- •
- Multiple-dose vials
The multiple-dose vial of RETACRIT contains the preservative benzyl alcohol. Benzyl alcohol has been shown to cause brain damage, other serious side effects and death in newborn and premature babies. RETACRIT that comes in single-dose vials does not contain benzyl alcohol.
Important: Follow these instructions exactly to help avoid infections.
Preparing the dose:
- 1.
- Remove the vial of RETACRIT from the refrigerator. During this time, protect the solution from light.
- 2.
- Do not use a single-dose vial of RETACRIT more than one time.
- 3.
- Do not shake RETACRIT.
- 4.
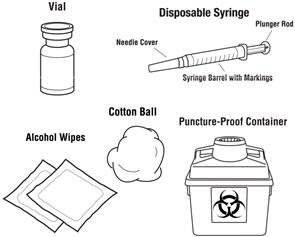
- Gather the other supplies you will need for your injection (vial, syringe, alcohol wipes, cotton ball, and a puncture-proof container for throwing away the syringe and needle). See Figure 1.
Figure 1
- 5.
- Check the date on the RETACRIT vial to be sure that the drug has not expired.
- 6.
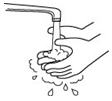
- Wash your hands well with soap and water before preparing the medicine. See Figure 2.
Figure 2
- 7.
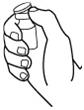
- Flip off the protective color cap on the top of the vial. Do not remove the grey rubber stopper. Wipe the top of the grey rubber stopper with an alcohol wipe. See Figures 3 and 4.
Figure 3 | Figure 4 |
- 8.
- Check the package containing the syringe. If the package has been opened or damaged, do not use that syringe. Throw away the syringe in the puncture-proof disposable container. If the syringe package is undamaged, open the package and remove the syringe.
- 9.
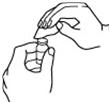
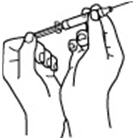
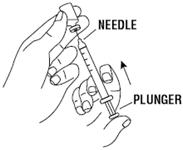
- Using a syringe and needle that has been recommended by your healthcare provider, carefully remove the needle cover. See Figure 5. Then draw air into the syringe by pulling back on the plunger. The amount of air drawn into the syringe should be equal to the amount (mL or cc) of the RETACRIT dose prescribed by your healthcare provider. See Figure 6.
Figure 5 | Figure 6 |
- 10.
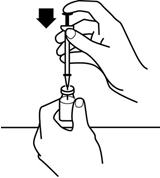
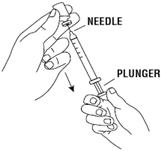
- With the vial on a flat work surface, insert the needle straight down through the grey rubber stopper of the RETACRIT vial. See Figure 7.
- 11.
- Push the plunger of the syringe down to inject the air from the syringe into the vial of RETACRIT. The air injected into the vial will allow RETACRIT to be easily withdrawn into the syringe. See Figure 7.
Figure 7
- 12.
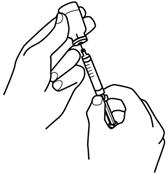
- Keep the needle inside the vial. Turn the vial and syringe upside down. Be sure the tip of the needle is in the RETACRIT liquid. Keep the vial upside down. Slowly pull back on the plunger to fill the syringe with RETACRIT liquid to the number (mL or cc) that matches the dose your healthcare provider prescribed. See Figure 8.
Figure 8
- 13.
- Keep the needle in the vial. Check for air bubbles in the syringe. A small amount of air is harmless. Too large an air bubble will give you the wrong RETACRIT dose. To remove air bubbles, gently tap the syringe with your fingers until the air bubbles rise to the top of the syringe. Slowly push the plunger up to force the air bubbles out of the syringe. Keep the tip of the needle in the RETACRIT liquid. Pull the plunger back to the number on the syringe that matches your dose. Check again for air bubbles. If there are still air bubbles, repeat the steps above to remove them. See Figures 9 and 10.
Figure 9 | Figure 10 |
- 14.
- Double-check that you have the correct dose in the syringe. Lay the vial down on its side with the needle still in it until after you have selected and prepared your site for injection.
Selecting and preparing the injection site:
RETACRIT can be injected into your body using two different ways (routes) as described below. Follow your healthcare provider's instructions about how you should inject RETACRIT. In patients on hemodialysis, the intravenous (IV) route is recommended.
- 1.Subcutaneous Route:
- •
- RETACRIT can be injected directly into a layer of fat under your skin. This is called a subcutaneous injection. When giving subcutaneous injections, follow your healthcare provider's instructions about changing the site for each injection. You may wish to write down the site where you have injected.
- •
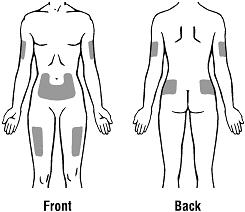
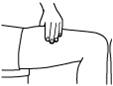
- Do not inject RETACRIT into an area that is tender, red, bruised, hard, or has scars or stretch marks. Recommended sites for injection are shown in Figure 11 below, including:
- o
- The outer area of the upper arms
- o
- The abdomen (except for the 2-inch area around the navel)
- o
- The front of the middle thighs
- o
- The upper outer area of the buttocks
Figure 11
- •
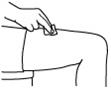
- Clean the skin with an alcohol wipe where the injection is to be made. Be careful not to touch the skin that has been wiped clean. See Figure 12.
Figure 12
- •
- Double-check that the correct amount of RETACRIT is in the syringe.
- •
- Remove the prepared syringe and needle from the vial of RETACRIT and hold it in the hand that you will use to inject the medicine.
- •
- Use the other hand to pinch a fold of skin at the cleaned injection site. Do not touch the cleaned area of skin. See Figure 13.
Figure 13
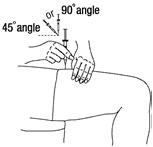
- •
- Hold the syringe like you would hold a pencil. Use a quick "dart-like" motion to insert the needle either straight up and down (90-degree angle) or at a slight angle (45 degrees) into the skin. Inject the prescribed dose subcutaneously as directed by your doctor, nurse or pharmacist. See Figure 14.
Figure 14
- •
- Pull the needle out of the skin and press a cotton ball or gauze over the injection site and hold it there for several seconds. Do not recap the needle.
- •
- Dispose of the used syringe and needle as described below. Do not reuse syringes and needles.
- 2.Intravenous Route:
- •
- RETACRIT can be injected in your vein through a special access port placed by your healthcare provider. This type of RETACRIT injection is called an intravenous (IV) injection. This route is usually for hemodialysis patients.
- •
- If you have a dialysis vascular access, make sure it is working by checking it as your healthcare provider has shown you. Be sure to let your healthcare provider know right away if you are having any problems, or if you have any questions.
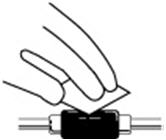
- •
- Wipe off the venous port of the hemodialysis tubing with an alcohol wipe. See Figure 15.
Figure 15
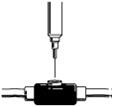
- •
- Insert the needle of the syringe into the cleaned venous port and push the plunger all the way down to inject all the RETACRIT. See Figure 16.
Figure 16
- •
- Remove the syringe from the venous port. Do not recap the needle.
- •
- Dispose of the used syringe and needle as described below.
How should I dispose of the vials, syringes, and needles?
Do not reuse the single-dose vials, syringes, or needles. Throw away the vials, syringes, and needles as instructed by your healthcare provider or by following these steps:
- •
- Do not throw the vials, syringes, or needles in the household trash or recycle.
- •
- Do not put the needle cover back on the needle.
- •
- Place all used needles and syringes in a puncture-proof disposable container with a lid. Do not use glass or clear plastic containers, or any container that will be recycled or returned to a store.
- •
- Keep the puncture-proof disposable container out of the reach of children.
- •
- When the puncture-proof disposable container is full, tape around the cap or lid to make sure the cap or lid does not come off. Throw away the puncture-proof disposable container as instructed by your healthcare provider. There may be special state and local laws for disposing of used needles and syringes. Do not throw the puncture-proof disposable container in the household trash. Do not recycle.
Keep RETACRIT and all medicines out of reach of children.
These Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured by Hospira, Inc., a Pfizer Company, Lake Forest, IL 60045 USA
US License No. 1974
Distributed by Pfizer Labs, a division of Pfizer Inc., New York, NY 10001 USA
LAB-0828-6.0
Revised: 4/2023
Find RETACRIT™ medical information:
Find RETACRIT™ medical information:
RETACRIT™ Quick Finder
Health Professional Information
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Inform patients:
- •
- Of the increased risks of mortality, serious cardiovascular reactions, thromboembolic reactions, stroke, and tumor progression [see Warnings and Precautions (5.1, 5.2)].
- •
- To undergo regular blood pressure monitoring, adhere to prescribed anti-hypertensive regimen and follow recommended dietary restrictions.
- •
- To contact their healthcare provider for new-onset neurologic symptoms or change in seizure frequency.
- •
- Of the need to have regular laboratory tests for hemoglobin.
- •
- Risks are associated with benzyl alcohol in neonates, infants, pregnant women, and lactating women [see Use in Specific Populations (8.1, 8.2, 8.4)].
Instruct patients who self-administer RETACRIT of the:
- •
- Importance of following the Instructions for Use.
- •
- Dangers of reusing needles, syringes, or unused portions of single-dose vials.
- •
- Proper disposal of used syringes, needles, and unused vials, and of the full container.
| This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 6/2024 | ||
MEDICATION GUIDE | ||
Read this Medication Guide:
This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment. Talk with your healthcare provider regularly about the use of RETACRIT and ask if there is new information about RETACRIT. | ||
What is the most important information I should know about RETACRIT? RETACRIT may cause serious side effects that can lead to death, including: For people with cancer:
For all people who take RETACRIT, including people with cancer or chronic kidney disease:
See "What are the possible side effects of RETACRIT?" below for more information. If you decide to take RETACRIT, your healthcare provider should prescribe the smallest dose of RETACRIT that is necessary to reduce your chance of needing RBC transfusions. | ||
What is RETACRIT? RETACRIT is a prescription medicine used to treat anemia. People with anemia have a lower-than-normal number of RBCs. RETACRIT works like the human protein called erythropoietin to help your body make more RBCs. RETACRIT is used to reduce or avoid the need for RBC transfusions. RETACRIT may be used to treat anemia if it is caused by:
RETACRIT may also be used to reduce the chance you will need RBC transfusions if you are scheduled for certain surgeries where a lot of blood loss is expected. If your hemoglobin level stays too high or if your hemoglobin goes up too quickly, this may lead to serious health problems which may result in death. These serious health problems may happen if you take RETACRIT, even if you do not have an increase in your hemoglobin level. RETACRIT has not been proven to improve quality of life, fatigue, or well-being. RETACRIT should not be used for treatment of anemia:
RETACRIT should not be used to reduce the chance you will need RBC transfusions if:
It is not known if RETACRIT is safe and effective in treating anemia in children less than 1 month old who have chronic kidney disease and in children less than 5 years old who have anemia caused by chemotherapy. | ||
Do not take RETACRIT if you:
Do not give RETACRIT from multiple-dose vials to:
| ||
Before taking RETACRIT, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | ||
How should I take RETACRIT?
| ||
What are the possible side effects of RETACRIT? RETACRIT may cause serious side effects, including:
Common side effects of RETACRIT include: | ||
|
|
|
These are not all of the possible side effects of RETACRIT. Your healthcare provider can give you a more complete list. Tell your healthcare provider about any side effects that bother you or that do not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | ||
How should I store RETACRIT?
Keep RETACRIT and all medicines out of the reach of children. | ||
General information about the safe and effective use of RETACRIT. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use RETACRIT for a condition for which it was not prescribed. Do not give RETACRIT to other people even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about RETACRIT that is written for healthcare professionals. | ||
What are the ingredients in RETACRIT? Active Ingredient: epoetin alfa-epbx Inactive Ingredients:
Manufactured by Hospira, Inc., a Pfizer Company, Lake Forest, IL 60045 USA  LAB-0827-9.0 | ||
Instructions for Use
RETACRIT (Ret-uh-krit)
(epoetin alfa-epbx)
Use these Instructions for Use if you or your caregiver has been trained to give RETACRIT injections at home. Do not give yourself the injection unless you have received training from your healthcare provider. If you are not sure about giving the injection or you have questions, ask your healthcare provider for help.
Before reading these Instructions for Use, read the Medication Guide that comes with RETACRIT for the most important information you need to know.
When you receive your RETACRIT vial make sure that:
- •
- The name RETACRIT appears on the carton and vial label.
- •
- The expiration date on the vial label has not passed. Do not use a vial of RETACRIT after the expiration date on the label.
- •
- The dose strength of the RETACRIT vial (number of Units per mL on the vial label) is the same as your healthcare provider prescribed.
- •
- You understand what the dose strength of RETACRIT means. RETACRIT vials come in several dose strengths. For example, the dose strength may be described as 10,000 Units/mL on the vial label. This strength means that 10,000 Units of medicine are contained in each 1 mL (milliliter) of liquid. Your healthcare provider may also refer to a mL as a "cc." One mL is the same as one "cc."
- •
- The RETACRIT liquid in the vial is clear and colorless. Do not use RETACRIT if the liquid in the vial looks discolored or cloudy, or if the liquid has lumps, flakes, or particles.
- •
- The RETACRIT vial has a color cap on the top of the vial. Do not use a vial of RETACRIT if the color cap on the top of the vial has been removed or is missing.
- •
- Use only the type of disposable syringe and needle that your healthcare provider has prescribed.
- •
- Do not shake RETACRIT. Shaking could cause RETACRIT not to work. If you shake RETACRIT, the solution in the vial may look foamy and should not be used.
- •
- Do not freeze RETACRIT. Do not use a vial of RETACRIT that has been frozen.
- •
- Do not use the carton of RETACRIT multiple-dose vials if it has been frozen or if the green area on the freeze strip indicator inside the RETACRIT carton looks white or cloudy.
- •
- Store RETACRIT in the refrigerator between 36°F to 46°F (2°C to 8°C).
- •
- Keep RETACRIT away from light.
- •
- Single-dose vials of RETACRIT should be used only one time. Throw the vial away after use even if there is medicine left in the vial.
- •
- After removing a dose from the multiple-dose vial, store the vial in the refrigerator (but not the freezer). Do not store the vial for more than 21 days.
- •
- Throw away the multiple-dose vials as directed by your healthcare provider:
- o
- if there is not enough medicine left in the multiple-dose vial for another dose, or
- o
- if it has been more than 21 days since you first put a needle into the multiple-dose vial.
How should I prepare for an injection of RETACRIT?
- •
- Always keep an extra syringe and needle on hand.
- •
- Follow your healthcare provider's instructions on how to measure your dose of RETACRIT. This dose will be measured in Units per mL or cc (1 mL is the same as 1 cc). Use a syringe that is marked in tenths of mL (for example, 0.2 mL or 0.2 cc). Using the wrong syringe can lead to a mistake in your dose and you could inject too much or too little RETACRIT.
Only use disposable syringes and needles. Use the syringes and needles only one time and then throw them away as instructed by your healthcare provider.
What do I need to know about the different types of RETACRIT vials?
RETACRIT comes in two different types of vials:
- •
- Single-dose vials
- •
- Multiple-dose vials
The multiple-dose vial of RETACRIT contains the preservative benzyl alcohol. Benzyl alcohol has been shown to cause brain damage, other serious side effects and death in newborn and premature babies. RETACRIT that comes in single-dose vials does not contain benzyl alcohol.
Important: Follow these instructions exactly to help avoid infections.
Preparing the dose:
- 1.
- Remove the vial of RETACRIT from the refrigerator. During this time, protect the solution from light.
- 2.
- Do not use a single-dose vial of RETACRIT more than one time.
- 3.
- Do not shake RETACRIT.
- 4.
- Gather the other supplies you will need for your injection (vial, syringe, alcohol wipes, cotton ball, and a puncture-proof container for throwing away the syringe and needle). See Figure 1.
Figure 1
- 5.
- Check the date on the RETACRIT vial to be sure that the drug has not expired.
- 6.
- Wash your hands well with soap and water before preparing the medicine. See Figure 2.
Figure 2
- 7.
- Flip off the protective color cap on the top of the vial. Do not remove the grey rubber stopper. Wipe the top of the grey rubber stopper with an alcohol wipe. See Figures 3 and 4.
Figure 3 | Figure 4 |
- 8.
- Check the package containing the syringe. If the package has been opened or damaged, do not use that syringe. Throw away the syringe in the puncture-proof disposable container. If the syringe package is undamaged, open the package and remove the syringe.
- 9.
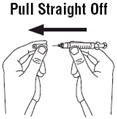
- Using a syringe and needle that has been recommended by your healthcare provider, carefully remove the needle cover. See Figure 5. Then draw air into the syringe by pulling back on the plunger. The amount of air drawn into the syringe should be equal to the amount (mL or cc) of the RETACRIT dose prescribed by your healthcare provider. See Figure 6.
Figure 5 | Figure 6 |
- 10.
- With the vial on a flat work surface, insert the needle straight down through the grey rubber stopper of the RETACRIT vial. See Figure 7.
- 11.
- Push the plunger of the syringe down to inject the air from the syringe into the vial of RETACRIT. The air injected into the vial will allow RETACRIT to be easily withdrawn into the syringe. See Figure 7.
Figure 7
- 12.
- Keep the needle inside the vial. Turn the vial and syringe upside down. Be sure the tip of the needle is in the RETACRIT liquid. Keep the vial upside down. Slowly pull back on the plunger to fill the syringe with RETACRIT liquid to the number (mL or cc) that matches the dose your healthcare provider prescribed. See Figure 8.
Figure 8
- 13.
- Keep the needle in the vial. Check for air bubbles in the syringe. A small amount of air is harmless. Too large an air bubble will give you the wrong RETACRIT dose. To remove air bubbles, gently tap the syringe with your fingers until the air bubbles rise to the top of the syringe. Slowly push the plunger up to force the air bubbles out of the syringe. Keep the tip of the needle in the RETACRIT liquid. Pull the plunger back to the number on the syringe that matches your dose. Check again for air bubbles. If there are still air bubbles, repeat the steps above to remove them. See Figures 9 and 10.
Figure 9 | Figure 10 |
- 14.
- Double-check that you have the correct dose in the syringe. Lay the vial down on its side with the needle still in it until after you have selected and prepared your site for injection.
Selecting and preparing the injection site:
RETACRIT can be injected into your body using two different ways (routes) as described below. Follow your healthcare provider's instructions about how you should inject RETACRIT. In patients on hemodialysis, the intravenous (IV) route is recommended.
- 1.Subcutaneous Route:
- •
- RETACRIT can be injected directly into a layer of fat under your skin. This is called a subcutaneous injection. When giving subcutaneous injections, follow your healthcare provider's instructions about changing the site for each injection. You may wish to write down the site where you have injected.
- •
- Do not inject RETACRIT into an area that is tender, red, bruised, hard, or has scars or stretch marks. Recommended sites for injection are shown in Figure 11 below, including:
- o
- The outer area of the upper arms
- o
- The abdomen (except for the 2-inch area around the navel)
- o
- The front of the middle thighs
- o
- The upper outer area of the buttocks
Figure 11
- •
- Clean the skin with an alcohol wipe where the injection is to be made. Be careful not to touch the skin that has been wiped clean. See Figure 12.
Figure 12
- •
- Double-check that the correct amount of RETACRIT is in the syringe.
- •
- Remove the prepared syringe and needle from the vial of RETACRIT and hold it in the hand that you will use to inject the medicine.
- •
- Use the other hand to pinch a fold of skin at the cleaned injection site. Do not touch the cleaned area of skin. See Figure 13.
Figure 13
- •
- Hold the syringe like you would hold a pencil. Use a quick "dart-like" motion to insert the needle either straight up and down (90-degree angle) or at a slight angle (45 degrees) into the skin. Inject the prescribed dose subcutaneously as directed by your doctor, nurse or pharmacist. See Figure 14.
Figure 14
- •
- Pull the needle out of the skin and press a cotton ball or gauze over the injection site and hold it there for several seconds. Do not recap the needle.
- •
- Dispose of the used syringe and needle as described below. Do not reuse syringes and needles.
- 2.Intravenous Route:
- •
- RETACRIT can be injected in your vein through a special access port placed by your healthcare provider. This type of RETACRIT injection is called an intravenous (IV) injection. This route is usually for hemodialysis patients.
- •
- If you have a dialysis vascular access, make sure it is working by checking it as your healthcare provider has shown you. Be sure to let your healthcare provider know right away if you are having any problems, or if you have any questions.
- •
- Wipe off the venous port of the hemodialysis tubing with an alcohol wipe. See Figure 15.
Figure 15
- •
- Insert the needle of the syringe into the cleaned venous port and push the plunger all the way down to inject all the RETACRIT. See Figure 16.
Figure 16
- •
- Remove the syringe from the venous port. Do not recap the needle.
- •
- Dispose of the used syringe and needle as described below.
How should I dispose of the vials, syringes, and needles?
Do not reuse the single-dose vials, syringes, or needles. Throw away the vials, syringes, and needles as instructed by your healthcare provider or by following these steps:
- •
- Do not throw the vials, syringes, or needles in the household trash or recycle.
- •
- Do not put the needle cover back on the needle.
- •
- Place all used needles and syringes in a puncture-proof disposable container with a lid. Do not use glass or clear plastic containers, or any container that will be recycled or returned to a store.
- •
- Keep the puncture-proof disposable container out of the reach of children.
- •
- When the puncture-proof disposable container is full, tape around the cap or lid to make sure the cap or lid does not come off. Throw away the puncture-proof disposable container as instructed by your healthcare provider. There may be special state and local laws for disposing of used needles and syringes. Do not throw the puncture-proof disposable container in the household trash. Do not recycle.
Keep RETACRIT and all medicines out of reach of children.
These Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured by Hospira, Inc., a Pfizer Company, Lake Forest, IL 60045 USA
US License No. 1974
Distributed by Pfizer Labs, a division of Pfizer Inc., New York, NY 10001 USA
LAB-0828-6.0
Revised: 4/2023
Health Professional Information
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Inform patients:
- •
- Of the increased risks of mortality, serious cardiovascular reactions, thromboembolic reactions, stroke, and tumor progression [see Warnings and Precautions (5.1, 5.2)].
- •
- To undergo regular blood pressure monitoring, adhere to prescribed anti-hypertensive regimen and follow recommended dietary restrictions.
- •
- To contact their healthcare provider for new-onset neurologic symptoms or change in seizure frequency.
- •
- Of the need to have regular laboratory tests for hemoglobin.
- •
- Risks are associated with benzyl alcohol in neonates, infants, pregnant women, and lactating women [see Use in Specific Populations (8.1, 8.2, 8.4)].
Instruct patients who self-administer RETACRIT of the:
- •
- Importance of following the Instructions for Use.
- •
- Dangers of reusing needles, syringes, or unused portions of single-dose vials.
- •
- Proper disposal of used syringes, needles, and unused vials, and of the full container.
| This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 6/2024 | ||
MEDICATION GUIDE | ||
Read this Medication Guide:
This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment. Talk with your healthcare provider regularly about the use of RETACRIT and ask if there is new information about RETACRIT. | ||
What is the most important information I should know about RETACRIT? RETACRIT may cause serious side effects that can lead to death, including: For people with cancer:
For all people who take RETACRIT, including people with cancer or chronic kidney disease:
See "What are the possible side effects of RETACRIT?" below for more information. If you decide to take RETACRIT, your healthcare provider should prescribe the smallest dose of RETACRIT that is necessary to reduce your chance of needing RBC transfusions. | ||
What is RETACRIT? RETACRIT is a prescription medicine used to treat anemia. People with anemia have a lower-than-normal number of RBCs. RETACRIT works like the human protein called erythropoietin to help your body make more RBCs. RETACRIT is used to reduce or avoid the need for RBC transfusions. RETACRIT may be used to treat anemia if it is caused by:
RETACRIT may also be used to reduce the chance you will need RBC transfusions if you are scheduled for certain surgeries where a lot of blood loss is expected. If your hemoglobin level stays too high or if your hemoglobin goes up too quickly, this may lead to serious health problems which may result in death. These serious health problems may happen if you take RETACRIT, even if you do not have an increase in your hemoglobin level. RETACRIT has not been proven to improve quality of life, fatigue, or well-being. RETACRIT should not be used for treatment of anemia:
RETACRIT should not be used to reduce the chance you will need RBC transfusions if:
It is not known if RETACRIT is safe and effective in treating anemia in children less than 1 month old who have chronic kidney disease and in children less than 5 years old who have anemia caused by chemotherapy. | ||
Do not take RETACRIT if you:
Do not give RETACRIT from multiple-dose vials to:
| ||
Before taking RETACRIT, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | ||
How should I take RETACRIT?
| ||
What are the possible side effects of RETACRIT? RETACRIT may cause serious side effects, including:
Common side effects of RETACRIT include: | ||
|
|
|
These are not all of the possible side effects of RETACRIT. Your healthcare provider can give you a more complete list. Tell your healthcare provider about any side effects that bother you or that do not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | ||
How should I store RETACRIT?
Keep RETACRIT and all medicines out of the reach of children. | ||
General information about the safe and effective use of RETACRIT. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use RETACRIT for a condition for which it was not prescribed. Do not give RETACRIT to other people even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about RETACRIT that is written for healthcare professionals. | ||
What are the ingredients in RETACRIT? Active Ingredient: epoetin alfa-epbx Inactive Ingredients:
Manufactured by Hospira, Inc., a Pfizer Company, Lake Forest, IL 60045 USA  LAB-0827-9.0 | ||
Instructions for Use
RETACRIT (Ret-uh-krit)
(epoetin alfa-epbx)
Use these Instructions for Use if you or your caregiver has been trained to give RETACRIT injections at home. Do not give yourself the injection unless you have received training from your healthcare provider. If you are not sure about giving the injection or you have questions, ask your healthcare provider for help.
Before reading these Instructions for Use, read the Medication Guide that comes with RETACRIT for the most important information you need to know.
When you receive your RETACRIT vial make sure that:
- •
- The name RETACRIT appears on the carton and vial label.
- •
- The expiration date on the vial label has not passed. Do not use a vial of RETACRIT after the expiration date on the label.
- •
- The dose strength of the RETACRIT vial (number of Units per mL on the vial label) is the same as your healthcare provider prescribed.
- •
- You understand what the dose strength of RETACRIT means. RETACRIT vials come in several dose strengths. For example, the dose strength may be described as 10,000 Units/mL on the vial label. This strength means that 10,000 Units of medicine are contained in each 1 mL (milliliter) of liquid. Your healthcare provider may also refer to a mL as a "cc." One mL is the same as one "cc."
- •
- The RETACRIT liquid in the vial is clear and colorless. Do not use RETACRIT if the liquid in the vial looks discolored or cloudy, or if the liquid has lumps, flakes, or particles.
- •
- The RETACRIT vial has a color cap on the top of the vial. Do not use a vial of RETACRIT if the color cap on the top of the vial has been removed or is missing.
- •
- Use only the type of disposable syringe and needle that your healthcare provider has prescribed.
- •
- Do not shake RETACRIT. Shaking could cause RETACRIT not to work. If you shake RETACRIT, the solution in the vial may look foamy and should not be used.
- •
- Do not freeze RETACRIT. Do not use a vial of RETACRIT that has been frozen.
- •
- Do not use the carton of RETACRIT multiple-dose vials if it has been frozen or if the green area on the freeze strip indicator inside the RETACRIT carton looks white or cloudy.
- •
- Store RETACRIT in the refrigerator between 36°F to 46°F (2°C to 8°C).
- •
- Keep RETACRIT away from light.
- •
- Single-dose vials of RETACRIT should be used only one time. Throw the vial away after use even if there is medicine left in the vial.
- •
- After removing a dose from the multiple-dose vial, store the vial in the refrigerator (but not the freezer). Do not store the vial for more than 21 days.
- •
- Throw away the multiple-dose vials as directed by your healthcare provider:
- o
- if there is not enough medicine left in the multiple-dose vial for another dose, or
- o
- if it has been more than 21 days since you first put a needle into the multiple-dose vial.
How should I prepare for an injection of RETACRIT?
- •
- Always keep an extra syringe and needle on hand.
- •
- Follow your healthcare provider's instructions on how to measure your dose of RETACRIT. This dose will be measured in Units per mL or cc (1 mL is the same as 1 cc). Use a syringe that is marked in tenths of mL (for example, 0.2 mL or 0.2 cc). Using the wrong syringe can lead to a mistake in your dose and you could inject too much or too little RETACRIT.
Only use disposable syringes and needles. Use the syringes and needles only one time and then throw them away as instructed by your healthcare provider.
What do I need to know about the different types of RETACRIT vials?
RETACRIT comes in two different types of vials:
- •
- Single-dose vials
- •
- Multiple-dose vials
The multiple-dose vial of RETACRIT contains the preservative benzyl alcohol. Benzyl alcohol has been shown to cause brain damage, other serious side effects and death in newborn and premature babies. RETACRIT that comes in single-dose vials does not contain benzyl alcohol.
Important: Follow these instructions exactly to help avoid infections.
Preparing the dose:
- 1.
- Remove the vial of RETACRIT from the refrigerator. During this time, protect the solution from light.
- 2.
- Do not use a single-dose vial of RETACRIT more than one time.
- 3.
- Do not shake RETACRIT.
- 4.
- Gather the other supplies you will need for your injection (vial, syringe, alcohol wipes, cotton ball, and a puncture-proof container for throwing away the syringe and needle). See Figure 1.
Figure 1
- 5.
- Check the date on the RETACRIT vial to be sure that the drug has not expired.
- 6.
- Wash your hands well with soap and water before preparing the medicine. See Figure 2.
Figure 2
- 7.
- Flip off the protective color cap on the top of the vial. Do not remove the grey rubber stopper. Wipe the top of the grey rubber stopper with an alcohol wipe. See Figures 3 and 4.
Figure 3 | Figure 4 |
- 8.
- Check the package containing the syringe. If the package has been opened or damaged, do not use that syringe. Throw away the syringe in the puncture-proof disposable container. If the syringe package is undamaged, open the package and remove the syringe.
- 9.
- Using a syringe and needle that has been recommended by your healthcare provider, carefully remove the needle cover. See Figure 5. Then draw air into the syringe by pulling back on the plunger. The amount of air drawn into the syringe should be equal to the amount (mL or cc) of the RETACRIT dose prescribed by your healthcare provider. See Figure 6.
Figure 5 | Figure 6 |
- 10.
- With the vial on a flat work surface, insert the needle straight down through the grey rubber stopper of the RETACRIT vial. See Figure 7.
- 11.
- Push the plunger of the syringe down to inject the air from the syringe into the vial of RETACRIT. The air injected into the vial will allow RETACRIT to be easily withdrawn into the syringe. See Figure 7.
Figure 7
- 12.
- Keep the needle inside the vial. Turn the vial and syringe upside down. Be sure the tip of the needle is in the RETACRIT liquid. Keep the vial upside down. Slowly pull back on the plunger to fill the syringe with RETACRIT liquid to the number (mL or cc) that matches the dose your healthcare provider prescribed. See Figure 8.
Figure 8
- 13.
- Keep the needle in the vial. Check for air bubbles in the syringe. A small amount of air is harmless. Too large an air bubble will give you the wrong RETACRIT dose. To remove air bubbles, gently tap the syringe with your fingers until the air bubbles rise to the top of the syringe. Slowly push the plunger up to force the air bubbles out of the syringe. Keep the tip of the needle in the RETACRIT liquid. Pull the plunger back to the number on the syringe that matches your dose. Check again for air bubbles. If there are still air bubbles, repeat the steps above to remove them. See Figures 9 and 10.
Figure 9 | Figure 10 |
- 14.
- Double-check that you have the correct dose in the syringe. Lay the vial down on its side with the needle still in it until after you have selected and prepared your site for injection.
Selecting and preparing the injection site:
RETACRIT can be injected into your body using two different ways (routes) as described below. Follow your healthcare provider's instructions about how you should inject RETACRIT. In patients on hemodialysis, the intravenous (IV) route is recommended.
- 1.Subcutaneous Route:
- •
- RETACRIT can be injected directly into a layer of fat under your skin. This is called a subcutaneous injection. When giving subcutaneous injections, follow your healthcare provider's instructions about changing the site for each injection. You may wish to write down the site where you have injected.
- •
- Do not inject RETACRIT into an area that is tender, red, bruised, hard, or has scars or stretch marks. Recommended sites for injection are shown in Figure 11 below, including:
- o
- The outer area of the upper arms
- o
- The abdomen (except for the 2-inch area around the navel)
- o
- The front of the middle thighs
- o
- The upper outer area of the buttocks
Figure 11
- •
- Clean the skin with an alcohol wipe where the injection is to be made. Be careful not to touch the skin that has been wiped clean. See Figure 12.
Figure 12
- •
- Double-check that the correct amount of RETACRIT is in the syringe.
- •
- Remove the prepared syringe and needle from the vial of RETACRIT and hold it in the hand that you will use to inject the medicine.
- •
- Use the other hand to pinch a fold of skin at the cleaned injection site. Do not touch the cleaned area of skin. See Figure 13.
Figure 13
- •
- Hold the syringe like you would hold a pencil. Use a quick "dart-like" motion to insert the needle either straight up and down (90-degree angle) or at a slight angle (45 degrees) into the skin. Inject the prescribed dose subcutaneously as directed by your doctor, nurse or pharmacist. See Figure 14.
Figure 14
- •
- Pull the needle out of the skin and press a cotton ball or gauze over the injection site and hold it there for several seconds. Do not recap the needle.
- •
- Dispose of the used syringe and needle as described below. Do not reuse syringes and needles.
- 2.Intravenous Route:
- •
- RETACRIT can be injected in your vein through a special access port placed by your healthcare provider. This type of RETACRIT injection is called an intravenous (IV) injection. This route is usually for hemodialysis patients.
- •
- If you have a dialysis vascular access, make sure it is working by checking it as your healthcare provider has shown you. Be sure to let your healthcare provider know right away if you are having any problems, or if you have any questions.
- •
- Wipe off the venous port of the hemodialysis tubing with an alcohol wipe. See Figure 15.
Figure 15
- •
- Insert the needle of the syringe into the cleaned venous port and push the plunger all the way down to inject all the RETACRIT. See Figure 16.
Figure 16
- •
- Remove the syringe from the venous port. Do not recap the needle.
- •
- Dispose of the used syringe and needle as described below.
How should I dispose of the vials, syringes, and needles?
Do not reuse the single-dose vials, syringes, or needles. Throw away the vials, syringes, and needles as instructed by your healthcare provider or by following these steps:
- •
- Do not throw the vials, syringes, or needles in the household trash or recycle.
- •
- Do not put the needle cover back on the needle.
- •
- Place all used needles and syringes in a puncture-proof disposable container with a lid. Do not use glass or clear plastic containers, or any container that will be recycled or returned to a store.
- •
- Keep the puncture-proof disposable container out of the reach of children.
- •
- When the puncture-proof disposable container is full, tape around the cap or lid to make sure the cap or lid does not come off. Throw away the puncture-proof disposable container as instructed by your healthcare provider. There may be special state and local laws for disposing of used needles and syringes. Do not throw the puncture-proof disposable container in the household trash. Do not recycle.
Keep RETACRIT and all medicines out of reach of children.
These Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured by Hospira, Inc., a Pfizer Company, Lake Forest, IL 60045 USA
US License No. 1974
Distributed by Pfizer Labs, a division of Pfizer Inc., New York, NY 10001 USA
LAB-0828-6.0
Revised: 4/2023
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information.9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.