OXBRYTA Full Patient Information
(voxelotor)
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Hypersensitivity Reactions
Advise patients that serious hypersensitivity reactions including DRESS may occur, and to notify their healthcare providers if they develop generalized rash, urticaria, shortness of breath, facial swelling, swelling around their eyes, lips, or tongue, fever, fatigue, muscle and/or joint pain, swollen glands, and eosinophilia [see Warnings and Precautions (5.1)]. Inform patients that some severe hypersensitivity reactions may affect their internal organs (such as liver, kidneys, lungs) and their blood cells. Advise patients to stop OXBRYTA if a severe hypersensitivity reaction is suspected.
Breastfeeding
Advise women not to breastfeed while they are on OXBRYTA therapy [see Use in Specific Populations (8.2)].
Dosage and Administration
To avoid a dosing error from using the wrong formulation of OXBRYTA, strongly advise patients and caregivers to visually inspect the tablets to verify the correct formulation each time the prescription is filled [see Dosage and Administration (2) and How Supplied/Storage and Handling (16)].
Advise patients to:
- •
- Store OXBRYTA at 20°C to 30°C (68°F to 86°F).
- •
- Continue taking OXBRYTA every day for as long as their physician tells them.
- •
- Do not take St John's wort while taking OXBRYTA.
- •
- Swallow OXBRYTA tablets whole. Do not cut, crush, or chew the tablets.
- •
- Do not swallow whole, cut, crush, or chew OXBRYTA tablets for oral suspension. Disperse OXBRYTA tablets for oral suspension in room temperature clear drink (such as drinking water, clear soda, apple juice, clear electrolyte drinks, clear flavored drinks, or clear sports drinks) before administration. The amount of liquid needed to disperse the tablets for oral suspension will depend on the dose (number of tablets prescribed) [see Dosage and Administration (2.7)].
- •
- Take OXBRYTA with or without food.
- •
- If a dose is missed or not consumed entirely, resume dosing the following day [see Dosage and Administration (2.7)].
This Patient Information has been approved by the U.S. Food and Drug Administration | Revised: 08/2023 | |||||
PATIENT INFORMATION | ||||||
OXBRYTA (ox brye ta) |
| OXBRYTA (ox brye ta) | ||||
What is OXBRYTA? | ||||||
Do not take OXBRYTA if you or your child have had an allergic reaction to voxelotor or any of the ingredients in OXBRYTA. See the end of this leaflet for a list of the ingredients in OXBRYTA. | ||||||
Before taking OXBRYTA, tell your healthcare provider about all of your medical conditions, including if you or your child:
Tell your healthcare provider about all the medicines you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may affect how OXBRYTA works. OXBRYTA may also affect how other medicines work and may affect the results of certain blood tests. Keep a list of all your medicines and show it to your healthcare provider. | ||||||
How should I take OXBRYTA?
| ||||||
What should I avoid while taking OXBRYTA? | ||||||
What are the possible side effects of OXBRYTA?
| ||||||
|
| |||||
The most common side effects of OXBRYTA include: | ||||||
|
| |||||
The most common side effects of OXBRYTA in children ages 4 to less than 12 years of age include: | ||||||
|
| |||||
These are not all the possible side effects of OXBRYTA. | ||||||
How should I store OXBRYTA?
Keep OXBRYTA and all medicines out of the reach of children. | ||||||
General information about the safe and effective use of OXBRYTA. | ||||||
What are the ingredients of OXBRYTA? Distributed by Global Blood Therapeutics, Inc A subsidiary of Pfizer Inc. South San Francisco, CA 94080 For more information, go to www.pfizer.com or call 1-800-438-1985. | ||||||
This Instructions for Use has been approved by the U.S. Food and Drug Administration. | Revised: 08/2023 | |||
INSTRUCTIONS FOR USE | ||||
This Instructions for Use contains information on how to take OXBRYTA tablets for oral suspension. | ||||
Important Information You Need to Know Before Taking OXBRYTA Tablets for Oral Suspension:
| ||||
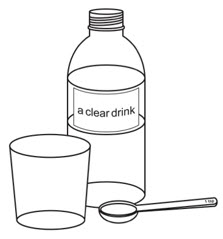
Gather supplies | ||||
You will need the following items to prepare the dose of OXBRYTA tablets for oral suspension (not included with OXBRYTA tablets for oral suspension):
You will also need:
|  | |||
Preparing a dose of OXBRYTA tablets for oral suspension | ||||
Step 1. | Wash and dry your hands well before preparing the dose. | |||
Step 2. | Pour room temperature clear drink into the cup. The table below shows the amount of clear drink needed for your prescribed dose. You may add more clear drink if needed to mix the tablets. | 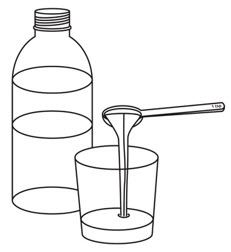 | ||
Number of OXBRYTA Tablets for Oral Suspension | Amount of Clear Drink | |||
1 | 1 teaspoon (5 mL) | |||
2 | 2 teaspoons (10 mL) | |||
3 | 3 teaspoons (15 mL) | |||
4 | 4 teaspoons (20 mL) | |||
5 | 5 teaspoons (25 mL) | |||
7 | 7 teaspoons (35 mL) | |||
8 | 8 teaspoons (40 mL) | |||
| ||||
Step 3. | Add the prescribed number of OXBRYTA tablets for oral suspension into the cup. | 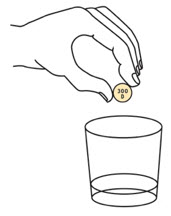 | ||
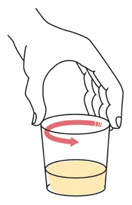
Step 4. | Swirl the cup until the tablet(s) break apart (disperse) in the drink. Be careful not to spill the mixture.
|  | ||
Step 5. | Wait for 1 to 5 minutes. |  | ||
Giving the dose | ||||
Step 6. | Swirl the cup again. Take or give all of the prepared medicine right away.
|  | ||
Step 7. | Add 1 or 2 teaspoons of room temperature clear drink to the cup to make sure the full dose is taken. Swirl the cup until the remaining medicine is mixed and take or give it right away.
|  | ||
Step 8. | Wash the teaspoon and cup with warm soap and water. | |||
Storing OXBRYTA
Keep OXBRYTA and all medicines out of the reach of children. | ||||
Disposing of OXBRYTA  Distributed by Global Blood Therapeutics, Inc A subsidiary of Pfizer Inc. South San Francisco, CA 94080 For more information, go to www.pfizer.com or call 1-800-438-1985. | ||||
Find OXBRYTA medical information:
Find OXBRYTA medical information:
OXBRYTA Quick Finder
Health Professional Information
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Hypersensitivity Reactions
Advise patients that serious hypersensitivity reactions including DRESS may occur, and to notify their healthcare providers if they develop generalized rash, urticaria, shortness of breath, facial swelling, swelling around their eyes, lips, or tongue, fever, fatigue, muscle and/or joint pain, swollen glands, and eosinophilia [see Warnings and Precautions (5.1)]. Inform patients that some severe hypersensitivity reactions may affect their internal organs (such as liver, kidneys, lungs) and their blood cells. Advise patients to stop OXBRYTA if a severe hypersensitivity reaction is suspected.
Breastfeeding
Advise women not to breastfeed while they are on OXBRYTA therapy [see Use in Specific Populations (8.2)].
Dosage and Administration
To avoid a dosing error from using the wrong formulation of OXBRYTA, strongly advise patients and caregivers to visually inspect the tablets to verify the correct formulation each time the prescription is filled [see Dosage and Administration (2) and How Supplied/Storage and Handling (16)].
Advise patients to:
- •
- Store OXBRYTA at 20°C to 30°C (68°F to 86°F).
- •
- Continue taking OXBRYTA every day for as long as their physician tells them.
- •
- Do not take St John's wort while taking OXBRYTA.
- •
- Swallow OXBRYTA tablets whole. Do not cut, crush, or chew the tablets.
- •
- Do not swallow whole, cut, crush, or chew OXBRYTA tablets for oral suspension. Disperse OXBRYTA tablets for oral suspension in room temperature clear drink (such as drinking water, clear soda, apple juice, clear electrolyte drinks, clear flavored drinks, or clear sports drinks) before administration. The amount of liquid needed to disperse the tablets for oral suspension will depend on the dose (number of tablets prescribed) [see Dosage and Administration (2.7)].
- •
- Take OXBRYTA with or without food.
- •
- If a dose is missed or not consumed entirely, resume dosing the following day [see Dosage and Administration (2.7)].
This Patient Information has been approved by the U.S. Food and Drug Administration | Revised: 08/2023 | |||||
PATIENT INFORMATION | ||||||
OXBRYTA (ox brye ta) |
| OXBRYTA (ox brye ta) | ||||
What is OXBRYTA? | ||||||
Do not take OXBRYTA if you or your child have had an allergic reaction to voxelotor or any of the ingredients in OXBRYTA. See the end of this leaflet for a list of the ingredients in OXBRYTA. | ||||||
Before taking OXBRYTA, tell your healthcare provider about all of your medical conditions, including if you or your child:
Tell your healthcare provider about all the medicines you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may affect how OXBRYTA works. OXBRYTA may also affect how other medicines work and may affect the results of certain blood tests. Keep a list of all your medicines and show it to your healthcare provider. | ||||||
How should I take OXBRYTA?
| ||||||
What should I avoid while taking OXBRYTA? | ||||||
What are the possible side effects of OXBRYTA?
| ||||||
|
| |||||
The most common side effects of OXBRYTA include: | ||||||
|
| |||||
The most common side effects of OXBRYTA in children ages 4 to less than 12 years of age include: | ||||||
|
| |||||
These are not all the possible side effects of OXBRYTA. | ||||||
How should I store OXBRYTA?
Keep OXBRYTA and all medicines out of the reach of children. | ||||||
General information about the safe and effective use of OXBRYTA. | ||||||
What are the ingredients of OXBRYTA? Distributed by Global Blood Therapeutics, Inc A subsidiary of Pfizer Inc. South San Francisco, CA 94080 For more information, go to www.pfizer.com or call 1-800-438-1985. | ||||||
This Instructions for Use has been approved by the U.S. Food and Drug Administration. | Revised: 08/2023 | |||
INSTRUCTIONS FOR USE | ||||
This Instructions for Use contains information on how to take OXBRYTA tablets for oral suspension. | ||||
Important Information You Need to Know Before Taking OXBRYTA Tablets for Oral Suspension:
| ||||
Gather supplies | ||||
You will need the following items to prepare the dose of OXBRYTA tablets for oral suspension (not included with OXBRYTA tablets for oral suspension):
You will also need:
|  | |||
Preparing a dose of OXBRYTA tablets for oral suspension | ||||
Step 1. | Wash and dry your hands well before preparing the dose. | |||
Step 2. | Pour room temperature clear drink into the cup. The table below shows the amount of clear drink needed for your prescribed dose. You may add more clear drink if needed to mix the tablets. |  | ||
Number of OXBRYTA Tablets for Oral Suspension | Amount of Clear Drink | |||
1 | 1 teaspoon (5 mL) | |||
2 | 2 teaspoons (10 mL) | |||
3 | 3 teaspoons (15 mL) | |||
4 | 4 teaspoons (20 mL) | |||
5 | 5 teaspoons (25 mL) | |||
7 | 7 teaspoons (35 mL) | |||
8 | 8 teaspoons (40 mL) | |||
| ||||
Step 3. | Add the prescribed number of OXBRYTA tablets for oral suspension into the cup. |  | ||
Step 4. | Swirl the cup until the tablet(s) break apart (disperse) in the drink. Be careful not to spill the mixture.
|  | ||
Step 5. | Wait for 1 to 5 minutes. |  | ||
Giving the dose | ||||
Step 6. | Swirl the cup again. Take or give all of the prepared medicine right away.
|  | ||
Step 7. | Add 1 or 2 teaspoons of room temperature clear drink to the cup to make sure the full dose is taken. Swirl the cup until the remaining medicine is mixed and take or give it right away.
|  | ||
Step 8. | Wash the teaspoon and cup with warm soap and water. | |||
Storing OXBRYTA
Keep OXBRYTA and all medicines out of the reach of children. | ||||
Disposing of OXBRYTA  Distributed by Global Blood Therapeutics, Inc A subsidiary of Pfizer Inc. South San Francisco, CA 94080 For more information, go to www.pfizer.com or call 1-800-438-1985. | ||||
Health Professional Information
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Hypersensitivity Reactions
Advise patients that serious hypersensitivity reactions including DRESS may occur, and to notify their healthcare providers if they develop generalized rash, urticaria, shortness of breath, facial swelling, swelling around their eyes, lips, or tongue, fever, fatigue, muscle and/or joint pain, swollen glands, and eosinophilia [see Warnings and Precautions (5.1)]. Inform patients that some severe hypersensitivity reactions may affect their internal organs (such as liver, kidneys, lungs) and their blood cells. Advise patients to stop OXBRYTA if a severe hypersensitivity reaction is suspected.
Breastfeeding
Advise women not to breastfeed while they are on OXBRYTA therapy [see Use in Specific Populations (8.2)].
Dosage and Administration
To avoid a dosing error from using the wrong formulation of OXBRYTA, strongly advise patients and caregivers to visually inspect the tablets to verify the correct formulation each time the prescription is filled [see Dosage and Administration (2) and How Supplied/Storage and Handling (16)].
Advise patients to:
- •
- Store OXBRYTA at 20°C to 30°C (68°F to 86°F).
- •
- Continue taking OXBRYTA every day for as long as their physician tells them.
- •
- Do not take St John's wort while taking OXBRYTA.
- •
- Swallow OXBRYTA tablets whole. Do not cut, crush, or chew the tablets.
- •
- Do not swallow whole, cut, crush, or chew OXBRYTA tablets for oral suspension. Disperse OXBRYTA tablets for oral suspension in room temperature clear drink (such as drinking water, clear soda, apple juice, clear electrolyte drinks, clear flavored drinks, or clear sports drinks) before administration. The amount of liquid needed to disperse the tablets for oral suspension will depend on the dose (number of tablets prescribed) [see Dosage and Administration (2.7)].
- •
- Take OXBRYTA with or without food.
- •
- If a dose is missed or not consumed entirely, resume dosing the following day [see Dosage and Administration (2.7)].
This Patient Information has been approved by the U.S. Food and Drug Administration | Revised: 08/2023 | |||||
PATIENT INFORMATION | ||||||
OXBRYTA (ox brye ta) |
| OXBRYTA (ox brye ta) | ||||
What is OXBRYTA? | ||||||
Do not take OXBRYTA if you or your child have had an allergic reaction to voxelotor or any of the ingredients in OXBRYTA. See the end of this leaflet for a list of the ingredients in OXBRYTA. | ||||||
Before taking OXBRYTA, tell your healthcare provider about all of your medical conditions, including if you or your child:
Tell your healthcare provider about all the medicines you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may affect how OXBRYTA works. OXBRYTA may also affect how other medicines work and may affect the results of certain blood tests. Keep a list of all your medicines and show it to your healthcare provider. | ||||||
How should I take OXBRYTA?
| ||||||
What should I avoid while taking OXBRYTA? | ||||||
What are the possible side effects of OXBRYTA?
| ||||||
|
| |||||
The most common side effects of OXBRYTA include: | ||||||
|
| |||||
The most common side effects of OXBRYTA in children ages 4 to less than 12 years of age include: | ||||||
|
| |||||
These are not all the possible side effects of OXBRYTA. | ||||||
How should I store OXBRYTA?
Keep OXBRYTA and all medicines out of the reach of children. | ||||||
General information about the safe and effective use of OXBRYTA. | ||||||
What are the ingredients of OXBRYTA? Distributed by Global Blood Therapeutics, Inc A subsidiary of Pfizer Inc. South San Francisco, CA 94080 For more information, go to www.pfizer.com or call 1-800-438-1985. | ||||||
This Instructions for Use has been approved by the U.S. Food and Drug Administration. | Revised: 08/2023 | |||
INSTRUCTIONS FOR USE | ||||
This Instructions for Use contains information on how to take OXBRYTA tablets for oral suspension. | ||||
Important Information You Need to Know Before Taking OXBRYTA Tablets for Oral Suspension:
| ||||
Gather supplies | ||||
You will need the following items to prepare the dose of OXBRYTA tablets for oral suspension (not included with OXBRYTA tablets for oral suspension):
You will also need:
|  | |||
Preparing a dose of OXBRYTA tablets for oral suspension | ||||
Step 1. | Wash and dry your hands well before preparing the dose. | |||
Step 2. | Pour room temperature clear drink into the cup. The table below shows the amount of clear drink needed for your prescribed dose. You may add more clear drink if needed to mix the tablets. |  | ||
Number of OXBRYTA Tablets for Oral Suspension | Amount of Clear Drink | |||
1 | 1 teaspoon (5 mL) | |||
2 | 2 teaspoons (10 mL) | |||
3 | 3 teaspoons (15 mL) | |||
4 | 4 teaspoons (20 mL) | |||
5 | 5 teaspoons (25 mL) | |||
7 | 7 teaspoons (35 mL) | |||
8 | 8 teaspoons (40 mL) | |||
| ||||
Step 3. | Add the prescribed number of OXBRYTA tablets for oral suspension into the cup. |  | ||
Step 4. | Swirl the cup until the tablet(s) break apart (disperse) in the drink. Be careful not to spill the mixture.
|  | ||
Step 5. | Wait for 1 to 5 minutes. |  | ||
Giving the dose | ||||
Step 6. | Swirl the cup again. Take or give all of the prepared medicine right away.
|  | ||
Step 7. | Add 1 or 2 teaspoons of room temperature clear drink to the cup to make sure the full dose is taken. Swirl the cup until the remaining medicine is mixed and take or give it right away.
|  | ||
Step 8. | Wash the teaspoon and cup with warm soap and water. | |||
Storing OXBRYTA
Keep OXBRYTA and all medicines out of the reach of children. | ||||
Disposing of OXBRYTA  Distributed by Global Blood Therapeutics, Inc A subsidiary of Pfizer Inc. South San Francisco, CA 94080 For more information, go to www.pfizer.com or call 1-800-438-1985. | ||||
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information.9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.
