NYVEPRIA Full Patient Information
(pegfilgrastim-apgf)
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Advise patients of the following risks and potential risks with NYVEPRIA:
- •
- Splenic rupture and splenomegaly
- •
- Acute Respiratory Distress Syndrome
- •
- Serious allergic reactions
- •
- Sickle cell crisis
- •
- Glomerulonephritis
- •
- Increased risk of Myelodysplastic Syndrome and/or Acute Myeloid Leukemia in patients with breast and lung cancer who receive pegfilgrastim products in conjunction with chemotherapy and/or radiation therapy
- •
- Capillary Leak Syndrome
- •
- Aortitis
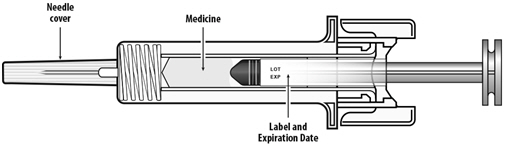
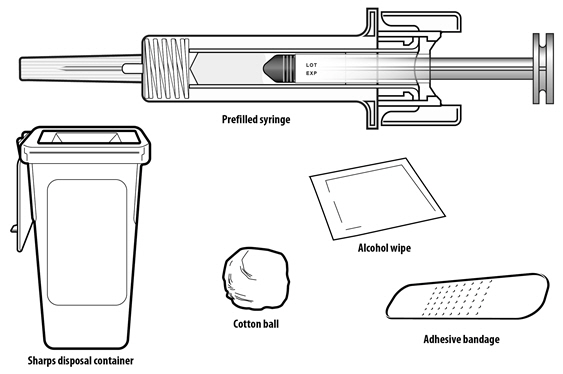
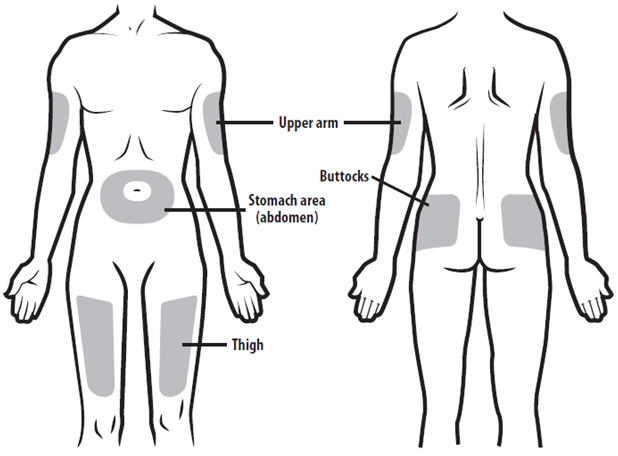
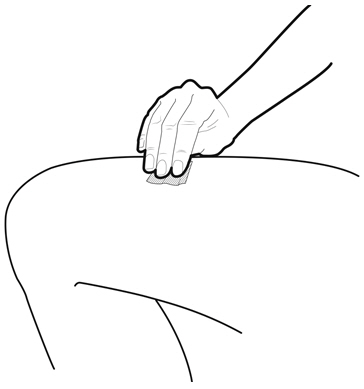
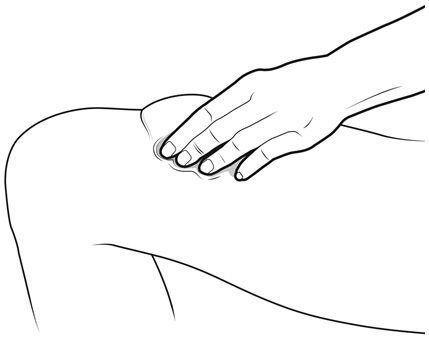
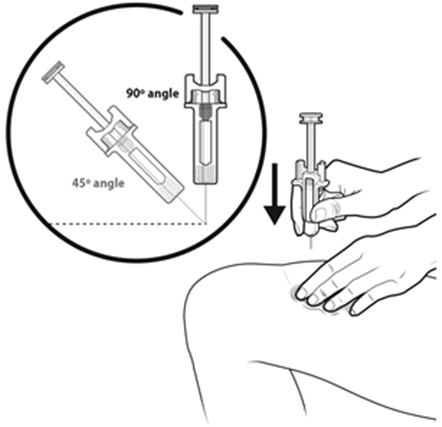
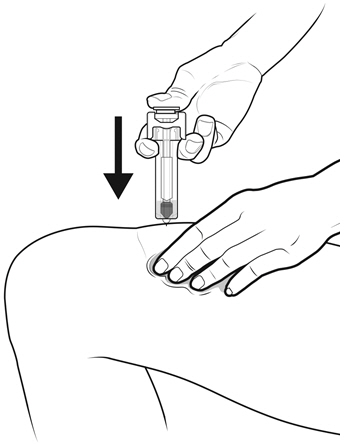
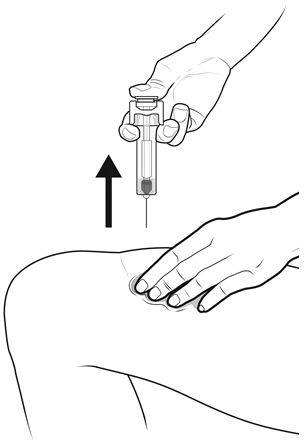
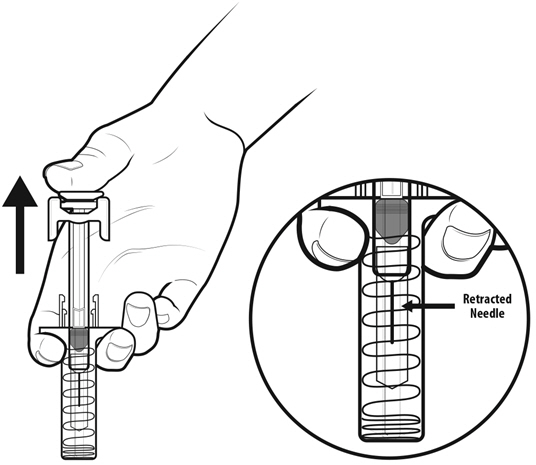
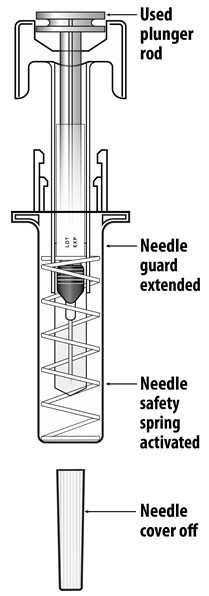
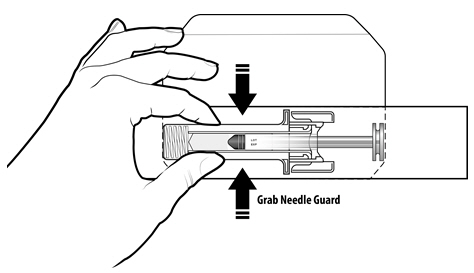
Instruct patients who self-administer NYVEPRIA using the single-dose prefilled syringe of the:
- •
- Importance of following the Instructions for Use.
- •
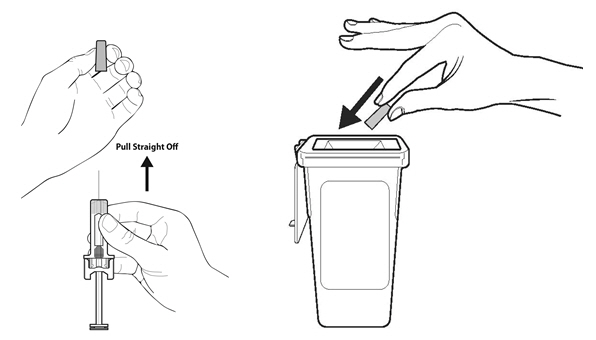
- Dangers of reusing syringes.
- •
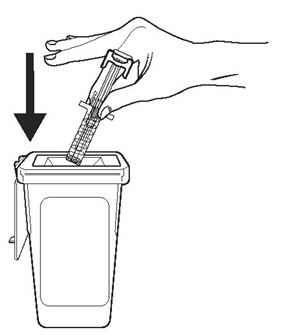
- Importance of following local requirements for proper disposal of used syringes.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
Find NYVEPRIA medical information:
Find NYVEPRIA medical information:
NYVEPRIA Quick Finder
Health Professional Information
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Advise patients of the following risks and potential risks with NYVEPRIA:
- •
- Splenic rupture and splenomegaly
- •
- Acute Respiratory Distress Syndrome
- •
- Serious allergic reactions
- •
- Sickle cell crisis
- •
- Glomerulonephritis
- •
- Increased risk of Myelodysplastic Syndrome and/or Acute Myeloid Leukemia in patients with breast and lung cancer who receive pegfilgrastim products in conjunction with chemotherapy and/or radiation therapy
- •
- Capillary Leak Syndrome
- •
- Aortitis
Instruct patients who self-administer NYVEPRIA using the single-dose prefilled syringe of the:
- •
- Importance of following the Instructions for Use.
- •
- Dangers of reusing syringes.
- •
- Importance of following local requirements for proper disposal of used syringes.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
Health Professional Information
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Advise patients of the following risks and potential risks with NYVEPRIA:
- •
- Splenic rupture and splenomegaly
- •
- Acute Respiratory Distress Syndrome
- •
- Serious allergic reactions
- •
- Sickle cell crisis
- •
- Glomerulonephritis
- •
- Increased risk of Myelodysplastic Syndrome and/or Acute Myeloid Leukemia in patients with breast and lung cancer who receive pegfilgrastim products in conjunction with chemotherapy and/or radiation therapy
- •
- Capillary Leak Syndrome
- •
- Aortitis
Instruct patients who self-administer NYVEPRIA using the single-dose prefilled syringe of the:
- •
- Importance of following the Instructions for Use.
- •
- Dangers of reusing syringes.
- •
- Importance of following local requirements for proper disposal of used syringes.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information.9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.

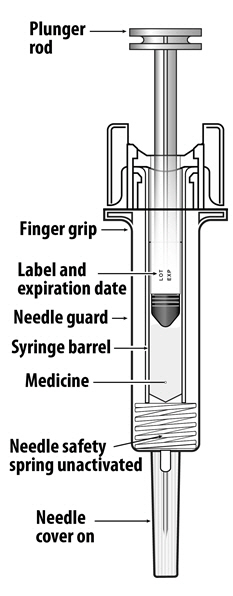
 For safety reasons:
For safety reasons: