CLEOCIN® Vaginal Cream
(clindamycin)
Find CLEOCIN® Vaginal Cream medical information:
Find CLEOCIN® Vaginal Cream medical information:
CLEOCIN® Vaginal Cream Quick Finder
DIRECTIONS FOR USE
DIRECTIONS FOR USE
Disposable plastic applicators are provided with this package. They are designed to allow proper vaginal administration of the cream.
Remove cap from cream tube. Screw a plastic applicator on the threaded end of the tube.
Rolling tube from the bottom, squeeze gently and force the medication into the applicator. The applicator is filled when the plunger reaches its predetermined stopping point.
Unscrew the applicator from the tube and replace the cap.
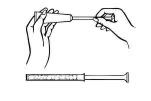
While lying on your back, firmly grasp the applicator barrel and insert into vagina as far as possible without causing discomfort.
Slowly push the plunger until it stops.
Carefully withdraw applicator from vagina, and discard applicator.
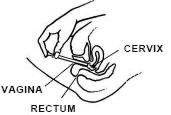
REMEMBER TO APPLY ONE APPLICATORFUL EACH NIGHT BEFORE BEDTIME, OR AS PRESCRIBED BY YOUR DOCTOR.
INSTRUCCIONES PARA LA PACIENTE
Este envase contiene aplicadores de plástico desechables. Los aplicadores están diseñados para la administración apropiada de la crema en la vagina.
Remueva la tapa del tubo de crema y enrosque el aplicador de plástico al tubo.
Exprima el tubo suavemente desde el extremo inferior y fuerce el medicamento al aplicador. El aplicador estará lleno cuando el émbolo llega a su máxima longitud.
Desenrosque el aplicador del tubo y vuelva a poner la tapa.
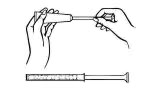
Acuéstese de espalda y agarrando firmemente el aplicador, introdúzcalo en la vagina tanto como sea posible sin causar molestias.
Empuje lentamente el émbolo hasta que se detenga.
Saque el aplicador cuidadosamente de la vagina y descártelo.
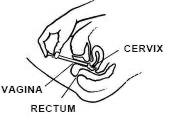
RECUERDE APLICARSE UN APLICADOR LLENO TODAS LAS NOCHES AL ACOSTARSE, O DE ACUERDO CON LAS INDICACIONES DE SU MEDICO.
LAB-1058-1.0
Indications and Usage
INDICATIONS AND USAGE
CLEOCIN Vaginal Cream 2%, is indicated in the treatment of bacterial vaginosis (formerly referred to as Haemophilus vaginitis, Gardnerella vaginitis, nonspecific vaginitis, Corynebacterium vaginitis, or anaerobic vaginosis). CLEOCIN Vaginal Cream 2%, can be used to treat non-pregnant women and pregnant women during the second and third trimester. (See CLINICAL STUDIES.)
NOTE: For purposes of this indication, a clinical diagnosis of bacterial vaginosis is usually defined by the presence of a homogeneous vaginal discharge that (a) has a pH of greater than 4.5, (b) emits a "fishy" amine odor when mixed with a 10% KOH solution, and (c) contains clue cells on microscopic examination. Gram's stain results consistent with a diagnosis of bacterial vaginosis include (a) markedly reduced or absent Lactobacillus morphology, (b) predominance of Gardnerella morphotype, and (c) absent or few white blood cells.
Other pathogens commonly associated with vulvovaginitis, eg, Trichomonas vaginalis, Chlamydia trachomatis, N. gonorrhoeae, Candida albicans, and Herpes simplex virus should be ruled out.
Dosage and Administration
DOSAGE AND ADMINISTRATION
The recommended dose is one applicatorful of clindamycin phosphate vaginal cream 2%, (5 grams containing approximately 100 mg of clindamycin phosphate) intravaginally, preferably at bedtime, for 3 or 7 consecutive days in non-pregnant patients and for 7 consecutive days in pregnant patients. (See CLINICAL STUDIES.)
Contraindications
CONTRAINDICATIONS
CLEOCIN Vaginal Cream 2%, is contraindicated in individuals with a history of hypersensitivity to clindamycin, lincomycin, or any of the components of this vaginal cream. CLEOCIN Vaginal Cream 2%, is also contraindicated in individuals with a history of regional enteritis, ulcerative colitis, or a history of "antibiotic-associated" colitis.
Warnings and Precautions
WARNINGS
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including clindamycin, and may range in severity from mild to life-threatening. Orally and parenterally administered clindamycin has been associated with severe colitis which may end fatally. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of orally and parenterally administered clindamycin, as well as with topical (dermal and vaginal) formulations of clindamycin. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of clindamycin, even when administered by the vaginal route, because approximately 5% of the clindamycin dose is systemically absorbed from the vagina.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia.Studies indicate that a toxin produced by Clostridioides difficile is a primary cause of "antibiotic-associated" colitis.
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to discontinuation of the drug alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridioides difficile colitis.
Onset of pseudomembranous colitis symptoms may occur during or after antimicrobial treatment.
PRECAUTIONS
General
CLEOCIN Vaginal Cream 2%, contains ingredients that will cause burning and irritation of the eye. In the event of accidental contact with the eye, rinse the eye with copious amounts of cool tap water.
The use of CLEOCIN Vaginal Cream 2% may result in the overgrowth of nonsusceptible organisms in the vagina. In clinical studies involving 600 non-pregnant women who received treatment for 3 days, Candida albicans was detected, either symptomatically or by culture, in 8.8% of patients. In 9% of the patients, vaginitis was recorded. Inclinical studies involving 1325 non-pregnant women who received treatment for 7 days, Candida albicans was detected, either symptomatically or by culture, in 10.5% of patients. Vaginitis was recorded in 10.7% of the patients. In 180 pregnant women who received treatment for 7 days, Candida albicans was detected, either symptomatically or by culture, in 13.3% of patients. In 7.2% of the patients, vaginitis was recorded. Candida albicans, as reported here, includes the terms: vaginal moniliasis and moniliasis (body as a whole). Vaginitis includes the terms: vulvovaginal disorder, vulvovaginitis, vaginal discharge, trichomonal vaginitis, and vaginitis.
Information for the Patient
The patient should be instructed not to engage in vaginal intercourse, or use other vaginal products (such as tampons or douches) during treatment with this product.
The patient should also be advised that this cream contains mineral oil that may weaken latex or rubber products such as condoms or vaginal contraceptive diaphragms. Therefore, use of such products within 72 hours following treatment with CLEOCIN Vaginal Cream 2%, is not recommended.
Drug Interactions
Systemic clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, it should be used with caution in patients receiving such agents.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals have not been performed with clindamycin to evaluate carcinogenic potential.
Genotoxicity tests performed included a rat micronucleus test and an Ames test. Both tests were negative. Fertility studies in rats treated orally with up to 300 mg/kg/day (31 times the human exposure based on mg/m2) revealed no effects on fertility or mating ability.
Pregnancy
Teratogenic effects
In clinical trials with pregnant women, the systemic administration of clindamycin during the second and third trimesters, has not been associated with an increased frequency of congenital abnormalities.
Clindamycin vaginal cream should be used during the first trimester of pregnancy only if clearly needed and the benefits outweigh the risks. There are no adequate and well-controlled studies in pregnant women during the first trimester of pregnancy.
CLEOCIN Vaginal Cream 2% has been studied in pregnant women during the second trimester. In women treated for seven days, abnormal labor was reported in 1.1% of patients who received clindamycin vaginal cream 2% compared with 0.5% of patients who received placebo.
Reproduction studies have been performed in rats and mice using oral and parenteral doses of clindamycin up to 600 mg/kg/day (62 and 25 times, respectively, the maximum human exposure based on body surface area) and have revealed no evidence of harm to the fetus due to clindamycin. Cleft palates were observed in fetuses from one mouse strain treated intraperitoneally with clindamycin at 200 mg/kg/day (about 10 times the recommended dose based on body surface area conversions). Since this effect was not observed in other mouse strains or in other species, the effect may be strain specific.
Nursing Mothers
Limited published data based on breast milk sampling reports that clindamycin appears in human breast milk in the range of less than 0.5 to 3.8 mcg/mL at dosages of 150 mg orally to 600 mg intravenously. It is not known if clindamycin is excreted in human breast milk following the use of vaginally administered clindamycin phosphate.
Clindamycin has the potential to cause adverse effects on the breast-fed infant's gastrointestinal flora. If clindamycin is required by a nursing mother, it is not a reason to discontinue breastfeeding, but an alternate drug may be preferred. Monitor the breast-fed infant for possible adverse effects on the gastrointestinal flora, such as diarrhea, candidiasis (thrush, diaper rash) or rarely, blood in the stool indicating possible antibiotic-associated colitis.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for clindamycin and any potential adverse effects on the breast-fed child from clindamycin or from the underlying maternal condition.
Geriatric Use
Clinical studies for CLEOCIN Vaginal Cream 2% did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Adverse Reactions
ADVERSE REACTIONS
Clinical trials
Non-pregnant Women
In clinical trials involving non-pregnant women, 1.8% of 600 patients who received treatment with CLEOCIN Vaginal Cream 2% for 3 days and 2.7% of 1325 patients who received treatment for 7 days discontinued therapy due to drug-related adverse events. Medical events judged to be related, probably related, possibly related, or of unknown relationship to vaginally administered clindamycin phosphate vaginal cream 2%, were reported for 20.7% of the patients receiving treatment for 3 days and 21.3% of the patients receiving treatment for 7 days. Events occurring in ≥1% of patients receiving clindamycin phosphate vaginal cream 2% are shown in Table 1.
| Event | CLEOCIN Vaginal Cream | |
|---|---|---|
| 3 Day n=600 | 7 Day n=1325 | |
| Urogenital | ||
| Vaginal moniliasis | 7.7 | 10.4 |
| Vulvovaginitis | 6.0 | 4.4 |
| Vulvovaginal disorder | 3.2 | 5.3 |
| Trichomonal vaginitis | 0 | 1.3 |
| Body as a Whole | ||
| Moniliasis (body) | 1.3 | 0.2 |
Other events occurring in <1% of the clindamycin vaginal cream 2% groups include:
Urogenital system: vaginal discharge, metrorrhagia, urinary tract infection, endometriosis, menstrual disorder, vaginitis/vaginal infection, and vaginal pain.
Body as a whole: localized abdominal pain, generalized abdominal pain, abdominal cramps, halitosis, headache, bacterial infection, inflammatory swelling, allergic reaction, and fungal infection.
Digestive system: nausea, vomiting, constipation, dyspepsia, flatulence, diarrhea, and gastrointestinal disorder.
Endocrine system: hyperthyroidism.
Central nervous system: dizziness and vertigo.
Respiratory system: epistaxis.
Skin: pruritus (non-application site), moniliasis, rash, maculopapular rash, erythema, and urticaria.
Special senses: taste perversion.
Pregnant Women
In a clinical trial involving pregnant women during the second trimester, 1.7% of 180 patients who received treatment for 7 days discontinued therapy due to drug-related adverse events. Medical events judged to be related, probably related, possibly related, or of unknown relationship to vaginally administered clindamycin phosphate vaginal cream 2%, were reported for 22.8% of pregnant patients. Events occurring in ≥1% of patients receiving either clindamycin phosphate vaginal cream 2% or placebo are shown in Table 2.
| Event | CLEOCIN Vaginal Cream | Placebo |
|---|---|---|
| 7 DAY n=180 | 7 DAY n=184 | |
| Urogenital | ||
| Vaginal moniliasis | 13.3 | 7.1 |
| Vulvovaginal disorder | 6.7 | 7.1 |
| Abnormal labor | 1.1 | 0.5 |
| Body as a Whole | ||
| Fungal infection | 1.7 | 0 |
| Skin | ||
| Pruritus, non-application site | 1.1 | 0 |
Other events occurring in <1% of the clindamycin vaginal cream 2% group include:
Urogenital system: dysuria, metrorrhagia, vaginal pain, and trichomonal vaginitis.
Body as a whole: upper respiratory infection.
Skin: pruritus (topical application site) and erythema.
Post-marketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In the post-marketing period, there have been case reports of pseudomembranous colitis with the use of clindamycin phosphate vaginal cream.
Other clindamycin formulations
Clindamycin vaginal cream affords minimal peak serum levels and systemic exposure (AUCs) of clindamycin compared to 100 mg oral clindamycin dosing. Although these lower levels of exposure are less likely to produce the common reactions seen with oral clindamycin, the possibility of these and other reactions cannot be excluded presently. Data from well-controlled trials directly comparing clindamycin administered orally to clindamycin administered vaginally are not available.
The following adverse reactions and altered laboratory tests have been reported with the oral or parenteral use of clindamycin:
Infections and Infestations: Clostridioides difficile colitis
Gastrointestinal: Abdominal pain, esophagitis, nausea, vomiting, diarrhea, and pseudomembranous colitis. (See WARNINGS.)
Hematopoietic: Transient neutropenia (leukopenia), eosinophilia, agranulocytosis, and thrombocytopenia have been reported. No direct etiologic relationship to concurrent clindamycin therapy could be made in any of these reports.
Hypersensitivity Reactions: Maculopapular rash and urticaria have been observed during drug therapy. Generalized mild to moderate morbilliform-like skin rashes are the most frequently reported of all adverse reactions. Cases of Acute Generalized Exanthematous Pustulosis (AGEP), erythema multiforme, some resembling Stevens-Johnson syndrome, have been associated with clindamycin. A few cases of anaphylactoid reactions have been reported. If a hypersensitivity reaction occurs, the drug should be discontinued.
Liver: Jaundice and abnormalities in liver function tests have been observed during clindamycin therapy.
Musculoskeletal: Cases of polyarthritis have been reported.
Renal: Acute kidney injury
Immune System: Drug reaction with eosinophilia and systemic symptoms (DRESS) cases have been reported.
Overdosage
OVERDOSAGE
Vaginally applied clindamycin phosphate vaginal cream 2% could be absorbed in sufficient amounts to produce systemic effects. (See WARNINGS.)
Description
DESCRIPTION
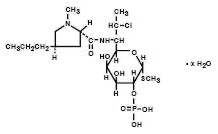
Clindamycin phosphate is a water soluble ester of the semi-synthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic lincomycin. The chemical name for clindamycin phosphate is methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α–D-galacto-octopyranoside 2-(dihydrogen phosphate). It has a molecular weight of 504.96, and the molecular formula is C18H34ClN2O8PS. The structural formula is represented below:
CLEOCIN Vaginal Cream 2%, is a semi-solid, white cream, which contains 2% clindamycin phosphate, USP, at a concentration equivalent to 20 mg clindamycin per gram. The pH of the cream is between 3.0 and 6.0. The cream also contains benzyl alcohol, cetostearyl alcohol, mixed fatty acid esters, mineral oil, polysorbate 60, propylene glycol, purified water, sorbitan monostearate, and stearic acid.
Each applicatorful of 5 grams of vaginal cream contains approximately 100 mg of clindamycin phosphate.
Clinical Pharmacology
CLINICAL PHARMACOLOGY
Pharmacokinetics
Following a once a day intravaginal dose of 100 mg of clindamycin phosphate vaginal cream 2%, administered to 6 healthy female volunteers for 7 days, approximately 5% (range 0.6% to 11%) of the administered dose was absorbed systemically. The peak serum clindamycin concentration observed on the first day averaged 18 ng/mL (range 4 to 47 ng/mL) and on day 7 it averaged 25 ng/mL (range 6 to 61 ng/mL). These peak concentrations were attained approximately 10 hours post-dosing (range 4–24 hours).
Following a once a day intravaginal dose of 100 mg of clindamycin phosphate vaginal cream 2%, administered for 7 consecutive days to 5 women with bacterial vaginosis, absorption was slower and less variable than that observed in healthy females. Approximately 5% (range 2% to 8%) of the dose was absorbed systemically. The peak serum clindamycin concentration observed on the first day averaged 13 ng/mL (range 6 to 34 ng/mL) and on day 7 it averaged 16 ng/mL (range 7 to 26 ng/mL). These peak concentrations were attained approximately 14 hours post-dosing (range 4–24 hours).
There was little or no systemic accumulation of clindamycin after repeated vaginal dosing of clindamycin phosphate vaginal cream 2%. The systemic half-life was 1.5 to 2.6 hours.
Clinical Studies
CLINICAL STUDIES
In two clinical studies involving 674 evaluable non-pregnant women with bacterial vaginosis comparing CLEOCIN Vaginal Cream 2% for 3 or 7 days, the clinical cure rates, determined at 1 month posttherapy, ranged from 72% to 81% for the 3-day treatment and 84% to 86% for the 7-day treatment.
| CLEOCIN 3 Day | CLEOCIN 7 Day | |||
|---|---|---|---|---|
| US Study | 94/131 | 72% | 110/128 | 86% |
| European Study | 161/199 | 81% | 181/216 | 84% |
In a clinical study involving 249 evaluable pregnant patients in the second and third trimester treated for 7 days, the clinical cure rate, determined at 1 month posttherapy, was 60% (77/129) in the clindamycin arm and 9% (11/120) for the vehicle arm. The determination of clinical cure was based on the absence of a "fishy" amine odor when the vaginal discharge was mixed with a 10% KOH solution and the absence of clue cells on microscopic examination.
How Supplied/Storage and Handling
Medication Guide
DIRECTIONS FOR USE
Disposable plastic applicators are provided with this package. They are designed to allow proper vaginal administration of the cream.
Remove cap from cream tube. Screw a plastic applicator on the threaded end of the tube.
Rolling tube from the bottom, squeeze gently and force the medication into the applicator. The applicator is filled when the plunger reaches its predetermined stopping point.
Unscrew the applicator from the tube and replace the cap.

While lying on your back, firmly grasp the applicator barrel and insert into vagina as far as possible without causing discomfort.
Slowly push the plunger until it stops.
Carefully withdraw applicator from vagina, and discard applicator.

REMEMBER TO APPLY ONE APPLICATORFUL EACH NIGHT BEFORE BEDTIME, OR AS PRESCRIBED BY YOUR DOCTOR.
INSTRUCCIONES PARA LA PACIENTE
Este envase contiene aplicadores de plástico desechables. Los aplicadores están diseñados para la administración apropiada de la crema en la vagina.
Remueva la tapa del tubo de crema y enrosque el aplicador de plástico al tubo.
Exprima el tubo suavemente desde el extremo inferior y fuerce el medicamento al aplicador. El aplicador estará lleno cuando el émbolo llega a su máxima longitud.
Desenrosque el aplicador del tubo y vuelva a poner la tapa.

Acuéstese de espalda y agarrando firmemente el aplicador, introdúzcalo en la vagina tanto como sea posible sin causar molestias.
Empuje lentamente el émbolo hasta que se detenga.
Saque el aplicador cuidadosamente de la vagina y descártelo.

RECUERDE APLICARSE UN APLICADOR LLENO TODAS LAS NOCHES AL ACOSTARSE, O DE ACUERDO CON LAS INDICACIONES DE SU MEDICO.
LAB-1058-1.0
Other
MICROBIOLOGY
Mechanism of Action
Clindamycin inhibits bacterial protein synthesis by binding to the 23S RNA of the 50S subunit of the ribosome. Clindamycin is predominantly bacteriostatic. Although clindamycin phosphate is inactive in vitro, rapid in vivo hydrolysis converts it to active clindamycin.
Resistance
Resistance to clindamycin is most often caused by modification of the target site on the ribosome, usually by chemical modification of RNA bases by point mutations in RNA or occasionally in proteins. Cross resistance has been demonstrated between lincosamides, macrolides and streptogramins B in some organisms. Cross resistance has been demonstrated between clindamycin and lincomycin.
Antibacterial Activity
Culture and sensitivity testing of bacteria are not routinely performed to establish the diagnosis of bacterial vaginosis (see INDICATIONS AND USAGE); standard methodology for the susceptibility testing of the potential bacterial pathogens, Gardnerella vaginalis, Mobiluncus spp., or Mycoplasma hominis, has not been defined.
The following in vitro data are available but their clinical significance is unknown. Clindamycin is active in vitro against most isolates of the following organisms reported to be associated with bacterial vaginosis:
- Bacteroides spp.
- Gardnerella vaginalis
- Mobiluncus spp.
- Mycoplasma hominis
- Peptostreptococcus spp.
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information. 9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.
