ABRILADA™ Full Patient Information
(adalimumab-afzb)
Full Patient Information
MEDICATION GUIDE | ||
Read the Medication Guide that comes with ABRILADA before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or treatment. | ||
What is the most important information I should know about ABRILADA?
You should not start taking ABRILADA if you have any kind of infection unless your healthcare provider says it is okay.
| ||
|
| |
After starting ABRILADA, call your healthcare provider right away if you have an infection, or any sign of an infection.
| ||
What is ABRILADA?
| ||
What should I tell my healthcare provider before taking ABRILADA?
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Keep a list of your medicines with you to show your healthcare provider and pharmacist each time you get a new medicine. | ||
How should I take ABRILADA?
| ||
What are the possible side effects of ABRILADA?
| ||
|
| |
| ||
|
| |
| ||
|
| |
| ||
|
| |
| ||
|
| |
Call your healthcare provider or get medical care right away if you develop any of the above symptoms. Your treatment with ABRILADA may be stopped.
These are not all of the possible side effects with ABRILADA. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. Ask your healthcare provider or pharmacist for more information. | ||
How should I store ABRILADA?
Keep ABRILADA, injection supplies, and all other medicines out of the reach of children. | ||
General information about the safe and effective use of ABRILADA | ||
What are the ingredients in ABRILADA? Active ingredient: adalimumab-afzb Inactive ingredients: edetate disodium dihydrate, L-histidine, L-histidine hydrochloride monohydrate, L-methionine, polysorbate 80, sucrose, and Water for Injection.  Manufactured by Pfizer Inc. For more information go to www.Pfizer.com. | ||
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 08/2023
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Infections
Inform patients that ABRILADA may lower the ability of their immune system to fight infections. Instruct patients of the importance of contacting their healthcare provider if they develop any symptoms of infection, including tuberculosis, invasive fungal infections, and reactivation of hepatitis B virus infections [see Warnings and Precautions (5.1, 5.2, 5.4)].
Malignancies
Counsel patients about the risk of malignancies while receiving ABRILADA [see Warnings and Precautions (5.2)].
Hypersensitivity Reactions
Advise patients to seek immediate medical attention if they experience any symptoms of severe hypersensitivity reactions [see Warnings and Precautions (5.3)].
Other Medical Conditions
Advise patients to report any signs of new or worsening medical conditions such as congestive heart failure, neurological disease, autoimmune disorders, or cytopenias. Advise patients to report any symptoms suggestive of a cytopenia such as bruising, bleeding, or persistent fever [see Warnings and Precautions (5.5, 5.6, 5.8, 5.9)].
Instructions on Injection Technique
Inform patients that the first injection is to be performed under the supervision of a qualified healthcare professional. If a patient or caregiver is to administer ABRILADA, instruct them in injection techniques and assess their ability to inject subcutaneously to ensure the proper administration of ABRILADA [see Instructions for Use].
For patients who will use ABRILADA pen, tell them that they:
- •
- Will only be able to press down the injection button when they are pushing the pen down firmly enough at the injection site.
- •
- Will hear a click when the injection button is pressed all the way down. The click means the start of the injection.
- •
- Will know the injection has finished and have received a full dose of medicine when they see the orange bar in the window, 5 seconds after hearing the 2nd click.
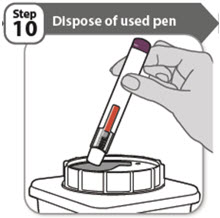
Instruct patients to dispose of their used needles and syringes or used pen in a FDA-cleared sharps disposal container immediately after use. Instruct patients not to dispose of loose needles and syringes or pen in their household trash. Instruct patients that if they do not have a FDA-cleared sharps disposal container, they may use a household container that is made of a heavy-duty plastic, can be closed with a tight-fitting and puncture-resistant lid without sharps being able to come out, upright and stable during use, leak-resistant, and properly labeled to warn of hazardous waste inside the container.
Instruct patients that when their sharps disposal container is almost full, they will need to follow their community guidelines for the correct way to dispose of their sharps disposal container. Instruct patients that there may be state or local laws regarding disposal of used needles and syringes. Refer patients to the FDA's website at http://www.fda.gov/safesharpsdisposal for more information about safe sharps disposal, and for specific information about sharps disposal in the state that they live in.
Instruct patients not to dispose of their used sharps disposal container in their household trash unless their community guidelines permit this. Instruct patients not to recycle their used sharps disposal container.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com. For Medical Information about ABRILADA, please visit www.pfizermedinfo.com or call 1-800-438-1985.
INSTRUCTIONS FOR USE
ABRILADA (AH brill-ah-dah)
(adalimumab-afzb)
40 mg/0.8 mL
Single-dose Prefilled Pen
Injection, for subcutaneous (under the skin) use
Keep this leaflet. These instructions show step by step directions on how to prepare and give an injection.
Storage Information:
- •
- Store your ABRILADA pen in the refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Store ABRILADA pen in the original carton until use to protect from light.
- •
- Do not freeze ABRILADA. Do not use ABRILADA if frozen, even if it has been thawed.
- •
- Refrigerated ABRILADA may be used until the expiration date printed on the ABRILADA carton or pen. Do not use ABRILADA after the expiration date.
- •
- If needed, for example when you are traveling, you may also store ABRILADA at room temperature up to 86°F (30°C) for up to 30 days. Store ABRILADA in the original carton until use to protect it from light.
- •
- Throw away ABRILADA if it has been kept at room temperature and not been used within 30 days.
- •
- Record the date you first remove ABRILADA from the refrigerator in the spaces provided on the ABRILADA pen carton.
- •
- Do not store ABRILADA in extreme heat or cold.
- •
- Do not use the pen if the liquid is cloudy, discolored, or has flakes or particles in it.
Keep ABRILADA, injection supplies, and all other medicines out of the reach of children.
ABRILADA for injection comes in a disposable (throw away) single-use pen that contains a single dose of medicine.
ABRILADA for injection can be given by a patient, caregiver or healthcare provider. Do not try to inject ABRILADA yourself until you are shown the right way to give the injections and read and understand the Instructions for Use. If your healthcare provider decides that you or a caregiver may be able to give your injections of ABRILADA at home, you should receive training on the right way to prepare and inject ABRILADA. It is important that you read, understand, and follow these instructions so that you inject ABRILADA the right way.
It is important to talk to your healthcare provider to be sure you understand your ABRILADA dosing instructions. To help you remember when to inject ABRILADA, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject ABRILADA.
Step 1. Supplies you need
- •
- You will need the following supplies for each injection of ABRILADA. Find a clean, flat surface to place the supplies on.
- o
- 1 ABRILADA pen (included inside the carton)
- o
- 1 alcohol swab (included inside the carton)
- o
- 1 cotton ball or gauze pad (not included in your ABRILADA carton)
- o
- 1 puncture resistant sharps disposal container for pen disposal (not included in your ABRILADA carton). See Step 10 "Dispose of used pen" at the end of this Instructions for Use.
Step 2. Getting ready
- •
- Remove the ABRILADA carton from the refrigerator.
- •
- Make sure the name ABRILADA appears on the carton and prefilled pen label.
- •
- Take out 1 ABRILADA pen and the alcohol swab. Keep your pen out of direct sunlight. Put the original carton with any unused pens back in the refrigerator.
- •
- Do not use your pen if:
- o
- your pen or the carton containing the pen has been dropped
- o
- it has been frozen or thawed
- o
- it has been kept in direct sunlight
- o
- it appears to be damaged
- o
- the seals on a new carton are broken
- o
- it has been out of the refrigerator for more than 30 days
- o
- the expiration date has passed
- o
- the liquid is cloudy, discolored or has flakes or particles
- •
- For a more comfortable injection, you may leave your pen at room temperature for 15 to 30 minutes before your injection.
- •
- Do not warm ABRILADA in any other way (for example, do not warm it in a microwave or in hot water).
- •
- Do not shake your pen. Shaking can damage your medicine.
- •
- Wash your hands with soap and water, and dry completely.
- •
- Do not remove the cap until you are ready to inject.
- •
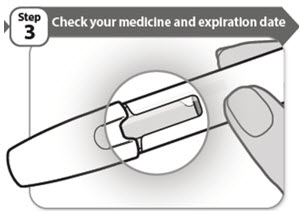
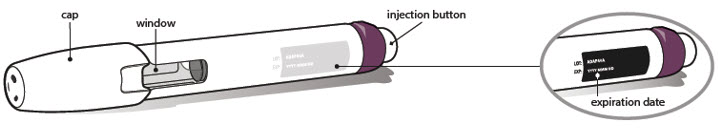
- Look carefully at your medicine in the window.
- •
- Make sure the medicine in the pen is clear and colorless to very light brown and free from flakes or particles.
- •
- It is normal to see one or more air bubbles in the window.
- •
- Check the expiration date on the pen label. The location of the expiration date on the pen label is shown below. Do not use the pen if the expiration date has passed.
If you have any questions about your medicine, please contact your healthcare provider or pharmacist.
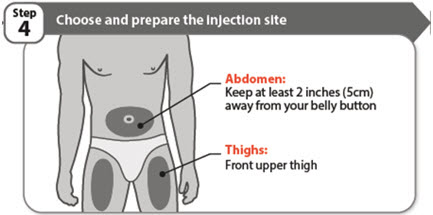
- •
- Choose a different injection site each time you give an injection:
- o
- Only use the front of your thighs or your lower abdomen (belly) as shown. If you choose your abdomen, do not use the area 2 inches around your belly button (navel).
- o
- Each new injection should be given at least one inch from a site you used before.
- •
- Do not inject into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- o
- If you have psoriasis, do not inject directly into any raised, thick, red, or scaly skin patches or lesions on your skin.
- •
- Do not inject through your clothes.
- •
- Wipe the injection site with the alcohol swab.
- •
- Allow the injection site to dry. Do not fan or blow on the clean area.
- •
- Do not touch this area again before giving the injection.
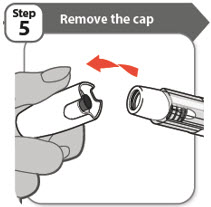
- •
- Twist and pull off the cap.
- •
- Throw the cap away into a sharps disposal container. You will not need it again.
Important: Handle your pen with care to avoid an accidental needle stick injury.
Note: A needle cover stays inside the cap after cap removal.
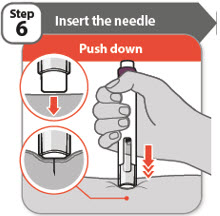
- •
- Push your pen firmly against the skin at 90 degrees, as shown in the diagram.
Note: The needle goes into the skin as you push your pen down. You will only be able to press down the injection button in Step 7 when you are pushing down firmly enough. - •
- Keep your pen pushed against the skin until Step 9.
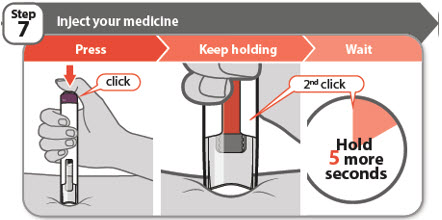
- •
- Press the injection button all the way down and you will hear a click. The click means the start of the injection.
- •
- Keep holding your pen firmly against the skin while the orange bar moves across the window. You will hear a 2nd click.
- •
- Wait for at least 5 more seconds after the 2nd click to make sure you get the full dose of medicine.
Note: If you cannot press down the injection button, it is because you are not pushing the pen down firmly enough at the injection site. See the Question and Answer section on the right side of this Instructions for Use for more information on what to do if the injection button does not press down.
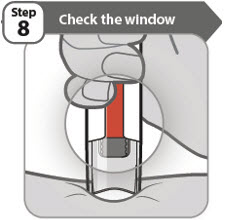
- •
- You should see an orange bar in the window.
- •
- Do not remove your pen until you have waited at least 5 seconds after the 2nd click and until the orange bar completely fills the window.
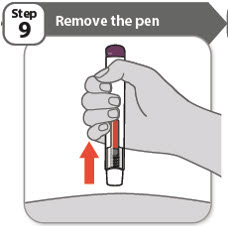
- •
- Remove your pen from the skin.
Note: After you remove your pen from the skin, the needle will be automatically covered. - •
- If the window has not turned orange, this means you have not received a full dose. Call your healthcare provider or pharmacist right away.
- •
- Do not inject another dose.
- •
- Put your used pen in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) pens in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of heavy duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- is properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
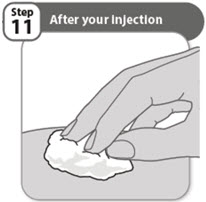
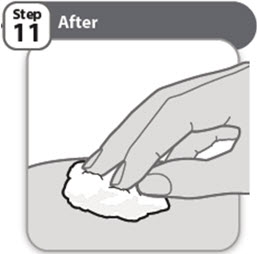
- •
- Look closely at your injection site. If there is blood, use a clean cotton ball or gauze pad to press lightly on the injection area for a few seconds.
- •
- Do not rub the injection site.
Note: Store any unused pens in the refrigerator in the original carton.
Questions and Answers
What should I do with my pen if it has been dropped?
Do not use it, even if it looks undamaged. Dispose of your pen in the same way as a used pen. You will need to use a new pen to give your injection.
Can I use my pen straight from the refrigerator?
Yes, however you may find that using the pen at room temperature reduces stinging or discomfort. If you allow your pen to reach room temperature before use, you must keep it away from direct sunlight as this can damage your medicine.
What should I do if I need to travel?
When you are traveling, you may store your pen in its carton at room temperature up to 86°F (30°C) for up to 30 days.
Is it okay to shake my pen before I use it?
No, do not shake your pen. Shaking can damage your medicine. When you check your medicine, gently tilt your pen back and forth while looking carefully into the window. It is normal to see one or more air bubbles.
Do I need to remove any air bubbles before using my pen?
No, do not attempt to remove air bubbles.
Drops of medicine have appeared at the needle tip. Is this okay?
Yes, it is normal to see a few drops of medicine at the needle tip when you remove the cap.
Can I re-insert the needle if I change my mind where I want to inject?
No, you should not re-insert the needle into your skin. If you change your mind, you will need a replacement pen if the needle has already been inserted into the skin. After the injection button has been pressed, you must not lift your pen from the skin until the injection has finished.
I pushed my pen against the skin but could not press the button down. What should I do?
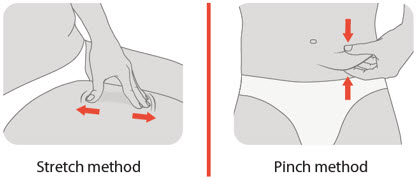
Take your finger off the injection button and push your pen down more firmly against the skin. Then try pushing the button again. If this does not work, stretching the skin may make the injection site firmer, making pressing the injection button easier.
Can I pinch or stretch the skin at the injection area?
Yes, pinching or stretching the skin before injection may make the injection site firmer, making it easier to press the injection button.
Do I need to keep my finger pressed on the injection button for the whole injection?
No, you can stop pressing the button when the injection has started. However, make sure you keep holding the pen firmly against the skin. The pen will continue to deliver your medicine.
How long will the injection take?
From the time the dose begins until you hear the 2nd click, it usually takes 3 to 10 seconds. After the 2nd click, you should continue to hold your pen in place for at least 5 more seconds to make sure you give the full dose.
What should I do if I see more than a small drop of medicine on the skin after giving my injection?
Nothing this time, but for your next injection wait a little longer before removing the pen from the skin to make sure all of the medicine went into your skin.
What should I do if I have any questions about my ABRILADA pen or medicine?
Contact your healthcare provider or pharmacist.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by Pfizer Inc.
New York, NY 10001
Distributed by Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
US License No. 2001
LAB-1352-3.0
Revised: 06/2023
INSTRUCTIONS FOR USE
ABRILADA (AH brill-ah-dah)
(adalimumab-afzb)
10 mg/0.2 mL, 20 mg/0.4 mL, 40 mg/0.8 mL
Single-dose Prefilled Syringe
Injection, for subcutaneous (under the skin) use only
Keep this leaflet. These instructions show step by step directions on how to prepare and give an injection.
Storage Information:
- •
- Store your ABRILADA prefilled syringe in the refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Store ABRILADA prefilled syringe in the original carton until use to protect from light.
- •
- Do not freeze ABRILADA. Do not use ABRILADA if frozen, even if it has been thawed.
- •
- Refrigerated ABRILADA may be used until the expiration date printed on the ABRILADA carton or prefilled syringe. Do not use ABRILADA after the expiration date.
- •
- If needed, for example when you are traveling, you may also store ABRILADA at room temperature up to 86°F (30°C) for up to 30 days. Store ABRILADA in the original carton until use to protect it from light.
- •
- Throw away ABRILADA if it has been kept at room temperature and not been used within 30 days.
- •
- Record the date you first remove ABRILADA from the refrigerator in the spaces provided on the ABRILADA prefilled syringe carton.
- •
- Do not store ABRILADA in extreme heat or cold.
- •
- Do not use a prefilled syringe if the liquid is cloudy, discolored, or has flakes or particles in it.
- •
- Do not drop or crush ABRILADA. The prefilled syringe is glass.
Keep ABRILADA, injection supplies, and all other medicines out of the reach of children.
ABRILADA for injection comes in a disposable (throw away) single use prefilled syringe that contains a single dose of medicine.
ABRILADA for injection can be given by a patient, caregiver or healthcare provider. Do not try to inject ABRILADA yourself until you are shown the right way to give the injections and read and understand the Instructions for Use. If your healthcare provider decides that you or a caregiver may be able to give your injections of ABRILADA at home, you should receive training on the right way to prepare and inject ABRILADA. It is important that you read, understand, and follow these instructions so that you inject ABRILADA the right way.
It is important to talk to your healthcare provider to be sure you understand your ABRILADA dosing instructions. To help you remember when to inject ABRILADA, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject ABRILADA.
Step 1. Supplies you need
- •
- You will need the following supplies for each injection of ABRILADA. Find a clean, flat surface to place the supplies on.
- o
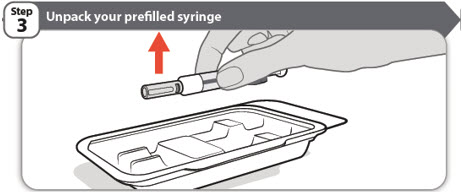
- 1 ABRILADA prefilled syringe in a tray inside the carton
- o
- 1 alcohol swab inside the carton
- o
- 1 cotton ball or gauze pad (not included in your ABRILADA carton)
- o
- 1 puncture resistant sharps disposal container for prefilled syringe disposal (not included in your ABRILADA carton). See Step 10 "Dispose of used syringe" at the end of this Instructions for Use.
Step 2. Getting ready
- •
- Remove ABRILADA carton from the refrigerator.
- •
- Open the carton and take out the tray containing your prefilled syringe.
- •
- Make sure the name ABRILADA appears on the dose tray and prefilled syringe label.
- •
- Check your carton and tray. Do not use if:
- o
- it has been dropped
- o
- it has been frozen or thawed
- o
- it appears to be damaged
- o
- the seals on a new carton are broken
- o
- it has been out of the refrigerator for more than 30 days
- o
- the expiration date has passed
Wash your hands with soap and water, and dry completely.
If you have any questions about your medicine, please contact your healthcare provider or pharmacist.
- •
- Peel back the paper seal on the tray.
- •
- Remove 1 prefilled syringe from the tray and put the original carton with any unused prefilled syringes back in the refrigerator.
- •
- Do not use your syringe if:
- o
- it appears to be damaged
- o
- It has been kept in direct sunlight
- o
- liquid is cloudy, discolored, or has flakes or particles
- •
- Do not shake your syringe. Shaking can damage your medicine.
- •
- For a more comfortable injection, leave the prefilled syringe at room temperature for 15 to 30 minutes before your injection.
- •
- Do not warm ABRILADA in any other way (for example, do not warm it in a microwave or in hot water).
- •
- Do not remove the needle cover from your prefilled syringe until you are ready to inject.
Always hold the prefilled syringe by the barrel to prevent damage.
- •
- Look carefully at your medicine in the window.
- •
- Make sure the medicine in the prefilled syringe is clear and colorless to very light brown and free from flakes or particles.
- •
- It is normal to see one or more air bubbles in the window.
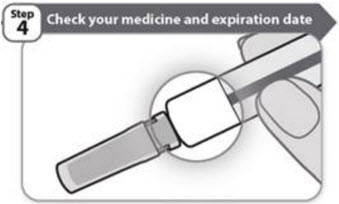
- •
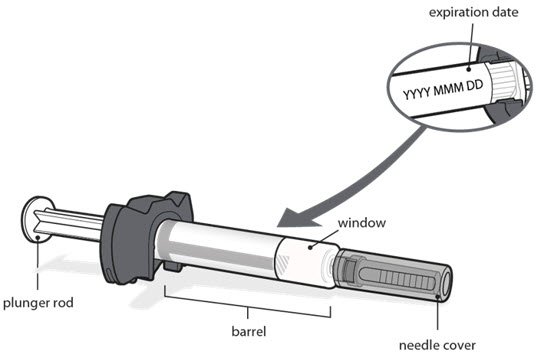
- Check the expiration date on the prefilled syringe label as shown in the figure in Step 1. Do not use the prefilled syringe if the expiration date has passed.
If you have any questions about your medicine, please contact your healthcare provider or pharmacist.
- •
- Choose a different injection site each time you give an injection.
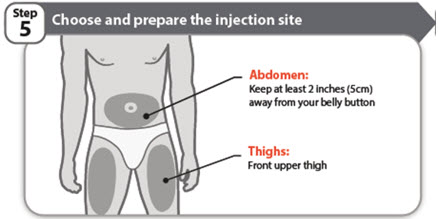
- o
- Only use the front of your thighs or your lower abdomen (belly) as shown. If you choose your abdomen, do not use the area 2 inches around your belly button (navel).
- o
- Each new injection should be given at least one inch from a site you used before.
- •
- Do not inject into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- o
- If you have psoriasis, do not inject directly into any raised, thick, red, or scaly skin patches or lesions on your skin.
- •
- Do not inject through your clothes.
- •
- Wipe the injection site with the alcohol swab.
- •
- Allow the injection site to dry. Do not fan or blow on the clean area.
- •
- Do not touch this area again before giving the injection.
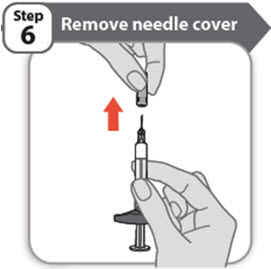
- •
- Hold the prefilled syringe by the barrel. Carefully pull the needle cover straight off and away from your body when you are ready to inject.
- •
- It is normal to see a drop of liquid at the end of the needle.
- •
- Throw the needle cover away into a sharps disposal container.
Note: Be careful when you handle your prefilled syringe to avoid an accidental needle stick injury.
- •
- Gently pinch up a fold of skin in the cleaned injection site area.
- •
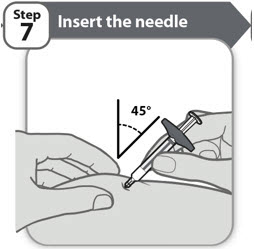
- Insert the needle to its full depth into the skin, at a 45 degree angle, as shown.
- •
- After the needle is inserted, release the pinched skin.
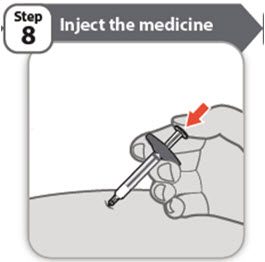
- •
- Using slow and constant pressure, push the plunger rod all the way down until the barrel is empty.
Note: It is recommended to hold your prefilled syringe in the skin for an additional 5 seconds after the plunger has been pressed down completely to make sure you get the full dose of medicine. - •
- Pull the needle out of the skin at the same angle at which it entered.
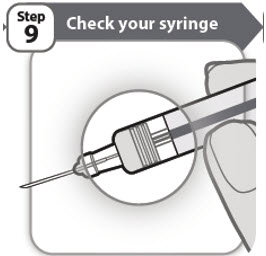
- •
- Check that your medicine has completely emptied from your prefilled syringe. If the gray stopper is not in the position shown, you may not have injected all of your medicine. Contact your healthcare provider right away.
- •
- Never re-insert the needle.
- •
- Never put the cap back on the needle.
- •
- Put your used syringe in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of heavy duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- is properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- •
- Look closely at your injection site. If there is blood, use a clean cotton ball or gauze pad to press lightly on the injection area for a few seconds.
- •
- Do not rub the site.
Note: Store any unused syringes in the refrigerator in the original carton.
Questions and Answers
What should I do with my prefilled syringe if it has been dropped?
Do not use it if it has been dropped or the carton containing your prefilled syringe has been dropped even if it looks undamaged. Dispose of your prefilled syringe in the same way as a used prefilled syringe. You will need to use a new prefilled syringe to give your injection.
Can I use my prefilled syringe straight from the refrigerator?
Yes, however you may find that using the prefilled syringe at room temperature reduces stinging or discomfort. If you allow your prefilled syringe to reach room temperature before use, you must keep it away from direct sunlight as this can damage your medicine.
What should I do if I need to travel?
When you are traveling, you may store your prefilled syringe in its carton at room temperature up to 86°F (30°C) for up to 30 days.
Is it okay to shake my prefilled syringe before I use it?
No, do not shake your prefilled syringe. Shaking can damage your medicine. When you check your medicine, gently tilt your syringe back and forth while looking carefully into the window. It is normal to see one or more bubbles.
Do I need to remove any air bubbles before using my prefilled syringe?
No, do not attempt to remove air bubbles.
Drops of medicine have appeared at the needle tip. Is this okay?
Yes, it is normal to see a few drops of medicine at the needle tip when you remove the needle cover.
Can I re-insert the needle into my skin?
No, you should not re-insert the needle into the skin. You will need a replacement prefilled syringe if the needle has already been inserted into the skin.
How long will the injection take?
Dose delivery will take approximately 2 to 5 seconds. Remember to hold your prefilled syringe in place for at least 5 seconds after the plunger has been pushed down all the way.
What should I do if I have any questions about my prefilled syringe or medicine?
Contact your healthcare provider or pharmacist.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by Pfizer Inc.
New York, NY 10001
Distributed by Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
US License No. 2001
LAB-1353-3.0
Revised: 06/2023
Find ABRILADA™ medical information:
Find ABRILADA™ medical information:
ABRILADA™ Quick Finder
Health Professional Information
Full Patient Information
Full Patient Information
MEDICATION GUIDE | ||
Read the Medication Guide that comes with ABRILADA before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or treatment. | ||
What is the most important information I should know about ABRILADA?
You should not start taking ABRILADA if you have any kind of infection unless your healthcare provider says it is okay.
| ||
|
| |
After starting ABRILADA, call your healthcare provider right away if you have an infection, or any sign of an infection.
| ||
What is ABRILADA?
| ||
What should I tell my healthcare provider before taking ABRILADA?
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Keep a list of your medicines with you to show your healthcare provider and pharmacist each time you get a new medicine. | ||
How should I take ABRILADA?
| ||
What are the possible side effects of ABRILADA?
| ||
|
| |
| ||
|
| |
| ||
|
| |
| ||
|
| |
| ||
|
| |
Call your healthcare provider or get medical care right away if you develop any of the above symptoms. Your treatment with ABRILADA may be stopped.
These are not all of the possible side effects with ABRILADA. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. Ask your healthcare provider or pharmacist for more information. | ||
How should I store ABRILADA?
Keep ABRILADA, injection supplies, and all other medicines out of the reach of children. | ||
General information about the safe and effective use of ABRILADA | ||
What are the ingredients in ABRILADA? Active ingredient: adalimumab-afzb Inactive ingredients: edetate disodium dihydrate, L-histidine, L-histidine hydrochloride monohydrate, L-methionine, polysorbate 80, sucrose, and Water for Injection.  Manufactured by Pfizer Inc. For more information go to www.Pfizer.com. | ||
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 08/2023
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Infections
Inform patients that ABRILADA may lower the ability of their immune system to fight infections. Instruct patients of the importance of contacting their healthcare provider if they develop any symptoms of infection, including tuberculosis, invasive fungal infections, and reactivation of hepatitis B virus infections [see Warnings and Precautions (5.1, 5.2, 5.4)].
Malignancies
Counsel patients about the risk of malignancies while receiving ABRILADA [see Warnings and Precautions (5.2)].
Hypersensitivity Reactions
Advise patients to seek immediate medical attention if they experience any symptoms of severe hypersensitivity reactions [see Warnings and Precautions (5.3)].
Other Medical Conditions
Advise patients to report any signs of new or worsening medical conditions such as congestive heart failure, neurological disease, autoimmune disorders, or cytopenias. Advise patients to report any symptoms suggestive of a cytopenia such as bruising, bleeding, or persistent fever [see Warnings and Precautions (5.5, 5.6, 5.8, 5.9)].
Instructions on Injection Technique
Inform patients that the first injection is to be performed under the supervision of a qualified healthcare professional. If a patient or caregiver is to administer ABRILADA, instruct them in injection techniques and assess their ability to inject subcutaneously to ensure the proper administration of ABRILADA [see Instructions for Use].
For patients who will use ABRILADA pen, tell them that they:
- •
- Will only be able to press down the injection button when they are pushing the pen down firmly enough at the injection site.
- •
- Will hear a click when the injection button is pressed all the way down. The click means the start of the injection.
- •
- Will know the injection has finished and have received a full dose of medicine when they see the orange bar in the window, 5 seconds after hearing the 2nd click.
Instruct patients to dispose of their used needles and syringes or used pen in a FDA-cleared sharps disposal container immediately after use. Instruct patients not to dispose of loose needles and syringes or pen in their household trash. Instruct patients that if they do not have a FDA-cleared sharps disposal container, they may use a household container that is made of a heavy-duty plastic, can be closed with a tight-fitting and puncture-resistant lid without sharps being able to come out, upright and stable during use, leak-resistant, and properly labeled to warn of hazardous waste inside the container.
Instruct patients that when their sharps disposal container is almost full, they will need to follow their community guidelines for the correct way to dispose of their sharps disposal container. Instruct patients that there may be state or local laws regarding disposal of used needles and syringes. Refer patients to the FDA's website at http://www.fda.gov/safesharpsdisposal for more information about safe sharps disposal, and for specific information about sharps disposal in the state that they live in.
Instruct patients not to dispose of their used sharps disposal container in their household trash unless their community guidelines permit this. Instruct patients not to recycle their used sharps disposal container.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com. For Medical Information about ABRILADA, please visit www.pfizermedinfo.com or call 1-800-438-1985.
INSTRUCTIONS FOR USE
ABRILADA (AH brill-ah-dah)
(adalimumab-afzb)
40 mg/0.8 mL
Single-dose Prefilled Pen
Injection, for subcutaneous (under the skin) use
Keep this leaflet. These instructions show step by step directions on how to prepare and give an injection.
Storage Information:
- •
- Store your ABRILADA pen in the refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Store ABRILADA pen in the original carton until use to protect from light.
- •
- Do not freeze ABRILADA. Do not use ABRILADA if frozen, even if it has been thawed.
- •
- Refrigerated ABRILADA may be used until the expiration date printed on the ABRILADA carton or pen. Do not use ABRILADA after the expiration date.
- •
- If needed, for example when you are traveling, you may also store ABRILADA at room temperature up to 86°F (30°C) for up to 30 days. Store ABRILADA in the original carton until use to protect it from light.
- •
- Throw away ABRILADA if it has been kept at room temperature and not been used within 30 days.
- •
- Record the date you first remove ABRILADA from the refrigerator in the spaces provided on the ABRILADA pen carton.
- •
- Do not store ABRILADA in extreme heat or cold.
- •
- Do not use the pen if the liquid is cloudy, discolored, or has flakes or particles in it.
Keep ABRILADA, injection supplies, and all other medicines out of the reach of children.
ABRILADA for injection comes in a disposable (throw away) single-use pen that contains a single dose of medicine.
ABRILADA for injection can be given by a patient, caregiver or healthcare provider. Do not try to inject ABRILADA yourself until you are shown the right way to give the injections and read and understand the Instructions for Use. If your healthcare provider decides that you or a caregiver may be able to give your injections of ABRILADA at home, you should receive training on the right way to prepare and inject ABRILADA. It is important that you read, understand, and follow these instructions so that you inject ABRILADA the right way.
It is important to talk to your healthcare provider to be sure you understand your ABRILADA dosing instructions. To help you remember when to inject ABRILADA, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject ABRILADA.
Step 1. Supplies you need
- •
- You will need the following supplies for each injection of ABRILADA. Find a clean, flat surface to place the supplies on.
- o
- 1 ABRILADA pen (included inside the carton)
- o
- 1 alcohol swab (included inside the carton)
- o
- 1 cotton ball or gauze pad (not included in your ABRILADA carton)
- o
- 1 puncture resistant sharps disposal container for pen disposal (not included in your ABRILADA carton). See Step 10 "Dispose of used pen" at the end of this Instructions for Use.
Step 2. Getting ready
- •
- Remove the ABRILADA carton from the refrigerator.
- •
- Make sure the name ABRILADA appears on the carton and prefilled pen label.
- •
- Take out 1 ABRILADA pen and the alcohol swab. Keep your pen out of direct sunlight. Put the original carton with any unused pens back in the refrigerator.
- •
- Do not use your pen if:
- o
- your pen or the carton containing the pen has been dropped
- o
- it has been frozen or thawed
- o
- it has been kept in direct sunlight
- o
- it appears to be damaged
- o
- the seals on a new carton are broken
- o
- it has been out of the refrigerator for more than 30 days
- o
- the expiration date has passed
- o
- the liquid is cloudy, discolored or has flakes or particles
- •
- For a more comfortable injection, you may leave your pen at room temperature for 15 to 30 minutes before your injection.
- •
- Do not warm ABRILADA in any other way (for example, do not warm it in a microwave or in hot water).
- •
- Do not shake your pen. Shaking can damage your medicine.
- •
- Wash your hands with soap and water, and dry completely.
- •
- Do not remove the cap until you are ready to inject.
- •
- Look carefully at your medicine in the window.
- •
- Make sure the medicine in the pen is clear and colorless to very light brown and free from flakes or particles.
- •
- It is normal to see one or more air bubbles in the window.
- •
- Check the expiration date on the pen label. The location of the expiration date on the pen label is shown below. Do not use the pen if the expiration date has passed.
If you have any questions about your medicine, please contact your healthcare provider or pharmacist.
- •
- Choose a different injection site each time you give an injection:
- o
- Only use the front of your thighs or your lower abdomen (belly) as shown. If you choose your abdomen, do not use the area 2 inches around your belly button (navel).
- o
- Each new injection should be given at least one inch from a site you used before.
- •
- Do not inject into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- o
- If you have psoriasis, do not inject directly into any raised, thick, red, or scaly skin patches or lesions on your skin.
- •
- Do not inject through your clothes.
- •
- Wipe the injection site with the alcohol swab.
- •
- Allow the injection site to dry. Do not fan or blow on the clean area.
- •
- Do not touch this area again before giving the injection.
- •
- Twist and pull off the cap.
- •
- Throw the cap away into a sharps disposal container. You will not need it again.
Important: Handle your pen with care to avoid an accidental needle stick injury.
Note: A needle cover stays inside the cap after cap removal.
- •
- Push your pen firmly against the skin at 90 degrees, as shown in the diagram.
Note: The needle goes into the skin as you push your pen down. You will only be able to press down the injection button in Step 7 when you are pushing down firmly enough. - •
- Keep your pen pushed against the skin until Step 9.
- •
- Press the injection button all the way down and you will hear a click. The click means the start of the injection.
- •
- Keep holding your pen firmly against the skin while the orange bar moves across the window. You will hear a 2nd click.
- •
- Wait for at least 5 more seconds after the 2nd click to make sure you get the full dose of medicine.
Note: If you cannot press down the injection button, it is because you are not pushing the pen down firmly enough at the injection site. See the Question and Answer section on the right side of this Instructions for Use for more information on what to do if the injection button does not press down.
- •
- You should see an orange bar in the window.
- •
- Do not remove your pen until you have waited at least 5 seconds after the 2nd click and until the orange bar completely fills the window.
- •
- Remove your pen from the skin.
Note: After you remove your pen from the skin, the needle will be automatically covered. - •
- If the window has not turned orange, this means you have not received a full dose. Call your healthcare provider or pharmacist right away.
- •
- Do not inject another dose.
- •
- Put your used pen in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) pens in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of heavy duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- is properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- •
- Look closely at your injection site. If there is blood, use a clean cotton ball or gauze pad to press lightly on the injection area for a few seconds.
- •
- Do not rub the injection site.
Note: Store any unused pens in the refrigerator in the original carton.
Questions and Answers
What should I do with my pen if it has been dropped?
Do not use it, even if it looks undamaged. Dispose of your pen in the same way as a used pen. You will need to use a new pen to give your injection.
Can I use my pen straight from the refrigerator?
Yes, however you may find that using the pen at room temperature reduces stinging or discomfort. If you allow your pen to reach room temperature before use, you must keep it away from direct sunlight as this can damage your medicine.
What should I do if I need to travel?
When you are traveling, you may store your pen in its carton at room temperature up to 86°F (30°C) for up to 30 days.
Is it okay to shake my pen before I use it?
No, do not shake your pen. Shaking can damage your medicine. When you check your medicine, gently tilt your pen back and forth while looking carefully into the window. It is normal to see one or more air bubbles.
Do I need to remove any air bubbles before using my pen?
No, do not attempt to remove air bubbles.
Drops of medicine have appeared at the needle tip. Is this okay?
Yes, it is normal to see a few drops of medicine at the needle tip when you remove the cap.
Can I re-insert the needle if I change my mind where I want to inject?
No, you should not re-insert the needle into your skin. If you change your mind, you will need a replacement pen if the needle has already been inserted into the skin. After the injection button has been pressed, you must not lift your pen from the skin until the injection has finished.
I pushed my pen against the skin but could not press the button down. What should I do?
Take your finger off the injection button and push your pen down more firmly against the skin. Then try pushing the button again. If this does not work, stretching the skin may make the injection site firmer, making pressing the injection button easier.
Can I pinch or stretch the skin at the injection area?
Yes, pinching or stretching the skin before injection may make the injection site firmer, making it easier to press the injection button.
Do I need to keep my finger pressed on the injection button for the whole injection?
No, you can stop pressing the button when the injection has started. However, make sure you keep holding the pen firmly against the skin. The pen will continue to deliver your medicine.
How long will the injection take?
From the time the dose begins until you hear the 2nd click, it usually takes 3 to 10 seconds. After the 2nd click, you should continue to hold your pen in place for at least 5 more seconds to make sure you give the full dose.
What should I do if I see more than a small drop of medicine on the skin after giving my injection?
Nothing this time, but for your next injection wait a little longer before removing the pen from the skin to make sure all of the medicine went into your skin.
What should I do if I have any questions about my ABRILADA pen or medicine?
Contact your healthcare provider or pharmacist.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by Pfizer Inc.
New York, NY 10001
Distributed by Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
US License No. 2001
LAB-1352-3.0
Revised: 06/2023
INSTRUCTIONS FOR USE
ABRILADA (AH brill-ah-dah)
(adalimumab-afzb)
10 mg/0.2 mL, 20 mg/0.4 mL, 40 mg/0.8 mL
Single-dose Prefilled Syringe
Injection, for subcutaneous (under the skin) use only
Keep this leaflet. These instructions show step by step directions on how to prepare and give an injection.
Storage Information:
- •
- Store your ABRILADA prefilled syringe in the refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Store ABRILADA prefilled syringe in the original carton until use to protect from light.
- •
- Do not freeze ABRILADA. Do not use ABRILADA if frozen, even if it has been thawed.
- •
- Refrigerated ABRILADA may be used until the expiration date printed on the ABRILADA carton or prefilled syringe. Do not use ABRILADA after the expiration date.
- •
- If needed, for example when you are traveling, you may also store ABRILADA at room temperature up to 86°F (30°C) for up to 30 days. Store ABRILADA in the original carton until use to protect it from light.
- •
- Throw away ABRILADA if it has been kept at room temperature and not been used within 30 days.
- •
- Record the date you first remove ABRILADA from the refrigerator in the spaces provided on the ABRILADA prefilled syringe carton.
- •
- Do not store ABRILADA in extreme heat or cold.
- •
- Do not use a prefilled syringe if the liquid is cloudy, discolored, or has flakes or particles in it.
- •
- Do not drop or crush ABRILADA. The prefilled syringe is glass.
Keep ABRILADA, injection supplies, and all other medicines out of the reach of children.
ABRILADA for injection comes in a disposable (throw away) single use prefilled syringe that contains a single dose of medicine.
ABRILADA for injection can be given by a patient, caregiver or healthcare provider. Do not try to inject ABRILADA yourself until you are shown the right way to give the injections and read and understand the Instructions for Use. If your healthcare provider decides that you or a caregiver may be able to give your injections of ABRILADA at home, you should receive training on the right way to prepare and inject ABRILADA. It is important that you read, understand, and follow these instructions so that you inject ABRILADA the right way.
It is important to talk to your healthcare provider to be sure you understand your ABRILADA dosing instructions. To help you remember when to inject ABRILADA, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject ABRILADA.
Step 1. Supplies you need
- •
- You will need the following supplies for each injection of ABRILADA. Find a clean, flat surface to place the supplies on.
- o
- 1 ABRILADA prefilled syringe in a tray inside the carton
- o
- 1 alcohol swab inside the carton
- o
- 1 cotton ball or gauze pad (not included in your ABRILADA carton)
- o
- 1 puncture resistant sharps disposal container for prefilled syringe disposal (not included in your ABRILADA carton). See Step 10 "Dispose of used syringe" at the end of this Instructions for Use.
Step 2. Getting ready
- •
- Remove ABRILADA carton from the refrigerator.
- •
- Open the carton and take out the tray containing your prefilled syringe.
- •
- Make sure the name ABRILADA appears on the dose tray and prefilled syringe label.
- •
- Check your carton and tray. Do not use if:
- o
- it has been dropped
- o
- it has been frozen or thawed
- o
- it appears to be damaged
- o
- the seals on a new carton are broken
- o
- it has been out of the refrigerator for more than 30 days
- o
- the expiration date has passed
Wash your hands with soap and water, and dry completely.
If you have any questions about your medicine, please contact your healthcare provider or pharmacist.
- •
- Peel back the paper seal on the tray.
- •
- Remove 1 prefilled syringe from the tray and put the original carton with any unused prefilled syringes back in the refrigerator.
- •
- Do not use your syringe if:
- o
- it appears to be damaged
- o
- It has been kept in direct sunlight
- o
- liquid is cloudy, discolored, or has flakes or particles
- •
- Do not shake your syringe. Shaking can damage your medicine.
- •
- For a more comfortable injection, leave the prefilled syringe at room temperature for 15 to 30 minutes before your injection.
- •
- Do not warm ABRILADA in any other way (for example, do not warm it in a microwave or in hot water).
- •
- Do not remove the needle cover from your prefilled syringe until you are ready to inject.
Always hold the prefilled syringe by the barrel to prevent damage.
- •
- Look carefully at your medicine in the window.
- •
- Make sure the medicine in the prefilled syringe is clear and colorless to very light brown and free from flakes or particles.
- •
- It is normal to see one or more air bubbles in the window.
- •
- Check the expiration date on the prefilled syringe label as shown in the figure in Step 1. Do not use the prefilled syringe if the expiration date has passed.
If you have any questions about your medicine, please contact your healthcare provider or pharmacist.
- •
- Choose a different injection site each time you give an injection.
- o
- Only use the front of your thighs or your lower abdomen (belly) as shown. If you choose your abdomen, do not use the area 2 inches around your belly button (navel).
- o
- Each new injection should be given at least one inch from a site you used before.
- •
- Do not inject into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- o
- If you have psoriasis, do not inject directly into any raised, thick, red, or scaly skin patches or lesions on your skin.
- •
- Do not inject through your clothes.
- •
- Wipe the injection site with the alcohol swab.
- •
- Allow the injection site to dry. Do not fan or blow on the clean area.
- •
- Do not touch this area again before giving the injection.
- •
- Hold the prefilled syringe by the barrel. Carefully pull the needle cover straight off and away from your body when you are ready to inject.
- •
- It is normal to see a drop of liquid at the end of the needle.
- •
- Throw the needle cover away into a sharps disposal container.
Note: Be careful when you handle your prefilled syringe to avoid an accidental needle stick injury.
- •
- Gently pinch up a fold of skin in the cleaned injection site area.
- •
- Insert the needle to its full depth into the skin, at a 45 degree angle, as shown.
- •
- After the needle is inserted, release the pinched skin.
- •
- Using slow and constant pressure, push the plunger rod all the way down until the barrel is empty.
Note: It is recommended to hold your prefilled syringe in the skin for an additional 5 seconds after the plunger has been pressed down completely to make sure you get the full dose of medicine. - •
- Pull the needle out of the skin at the same angle at which it entered.
- •
- Check that your medicine has completely emptied from your prefilled syringe. If the gray stopper is not in the position shown, you may not have injected all of your medicine. Contact your healthcare provider right away.
- •
- Never re-insert the needle.
- •
- Never put the cap back on the needle.
- •
- Put your used syringe in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of heavy duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- is properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- •
- Look closely at your injection site. If there is blood, use a clean cotton ball or gauze pad to press lightly on the injection area for a few seconds.
- •
- Do not rub the site.
Note: Store any unused syringes in the refrigerator in the original carton.
Questions and Answers
What should I do with my prefilled syringe if it has been dropped?
Do not use it if it has been dropped or the carton containing your prefilled syringe has been dropped even if it looks undamaged. Dispose of your prefilled syringe in the same way as a used prefilled syringe. You will need to use a new prefilled syringe to give your injection.
Can I use my prefilled syringe straight from the refrigerator?
Yes, however you may find that using the prefilled syringe at room temperature reduces stinging or discomfort. If you allow your prefilled syringe to reach room temperature before use, you must keep it away from direct sunlight as this can damage your medicine.
What should I do if I need to travel?
When you are traveling, you may store your prefilled syringe in its carton at room temperature up to 86°F (30°C) for up to 30 days.
Is it okay to shake my prefilled syringe before I use it?
No, do not shake your prefilled syringe. Shaking can damage your medicine. When you check your medicine, gently tilt your syringe back and forth while looking carefully into the window. It is normal to see one or more bubbles.
Do I need to remove any air bubbles before using my prefilled syringe?
No, do not attempt to remove air bubbles.
Drops of medicine have appeared at the needle tip. Is this okay?
Yes, it is normal to see a few drops of medicine at the needle tip when you remove the needle cover.
Can I re-insert the needle into my skin?
No, you should not re-insert the needle into the skin. You will need a replacement prefilled syringe if the needle has already been inserted into the skin.
How long will the injection take?
Dose delivery will take approximately 2 to 5 seconds. Remember to hold your prefilled syringe in place for at least 5 seconds after the plunger has been pushed down all the way.
What should I do if I have any questions about my prefilled syringe or medicine?
Contact your healthcare provider or pharmacist.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by Pfizer Inc.
New York, NY 10001
Distributed by Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
US License No. 2001
LAB-1353-3.0
Revised: 06/2023
Health Professional Information
Full Patient Information
Full Patient Information
MEDICATION GUIDE | ||
Read the Medication Guide that comes with ABRILADA before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or treatment. | ||
What is the most important information I should know about ABRILADA?
You should not start taking ABRILADA if you have any kind of infection unless your healthcare provider says it is okay.
| ||
|
| |
After starting ABRILADA, call your healthcare provider right away if you have an infection, or any sign of an infection.
| ||
What is ABRILADA?
| ||
What should I tell my healthcare provider before taking ABRILADA?
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Keep a list of your medicines with you to show your healthcare provider and pharmacist each time you get a new medicine. | ||
How should I take ABRILADA?
| ||
What are the possible side effects of ABRILADA?
| ||
|
| |
| ||
|
| |
| ||
|
| |
| ||
|
| |
| ||
|
| |
Call your healthcare provider or get medical care right away if you develop any of the above symptoms. Your treatment with ABRILADA may be stopped.
These are not all of the possible side effects with ABRILADA. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. Ask your healthcare provider or pharmacist for more information. | ||
How should I store ABRILADA?
Keep ABRILADA, injection supplies, and all other medicines out of the reach of children. | ||
General information about the safe and effective use of ABRILADA | ||
What are the ingredients in ABRILADA? Active ingredient: adalimumab-afzb Inactive ingredients: edetate disodium dihydrate, L-histidine, L-histidine hydrochloride monohydrate, L-methionine, polysorbate 80, sucrose, and Water for Injection.  Manufactured by Pfizer Inc. For more information go to www.Pfizer.com. | ||
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 08/2023
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Infections
Inform patients that ABRILADA may lower the ability of their immune system to fight infections. Instruct patients of the importance of contacting their healthcare provider if they develop any symptoms of infection, including tuberculosis, invasive fungal infections, and reactivation of hepatitis B virus infections [see Warnings and Precautions (5.1, 5.2, 5.4)].
Malignancies
Counsel patients about the risk of malignancies while receiving ABRILADA [see Warnings and Precautions (5.2)].
Hypersensitivity Reactions
Advise patients to seek immediate medical attention if they experience any symptoms of severe hypersensitivity reactions [see Warnings and Precautions (5.3)].
Other Medical Conditions
Advise patients to report any signs of new or worsening medical conditions such as congestive heart failure, neurological disease, autoimmune disorders, or cytopenias. Advise patients to report any symptoms suggestive of a cytopenia such as bruising, bleeding, or persistent fever [see Warnings and Precautions (5.5, 5.6, 5.8, 5.9)].
Instructions on Injection Technique
Inform patients that the first injection is to be performed under the supervision of a qualified healthcare professional. If a patient or caregiver is to administer ABRILADA, instruct them in injection techniques and assess their ability to inject subcutaneously to ensure the proper administration of ABRILADA [see Instructions for Use].
For patients who will use ABRILADA pen, tell them that they:
- •
- Will only be able to press down the injection button when they are pushing the pen down firmly enough at the injection site.
- •
- Will hear a click when the injection button is pressed all the way down. The click means the start of the injection.
- •
- Will know the injection has finished and have received a full dose of medicine when they see the orange bar in the window, 5 seconds after hearing the 2nd click.
Instruct patients to dispose of their used needles and syringes or used pen in a FDA-cleared sharps disposal container immediately after use. Instruct patients not to dispose of loose needles and syringes or pen in their household trash. Instruct patients that if they do not have a FDA-cleared sharps disposal container, they may use a household container that is made of a heavy-duty plastic, can be closed with a tight-fitting and puncture-resistant lid without sharps being able to come out, upright and stable during use, leak-resistant, and properly labeled to warn of hazardous waste inside the container.
Instruct patients that when their sharps disposal container is almost full, they will need to follow their community guidelines for the correct way to dispose of their sharps disposal container. Instruct patients that there may be state or local laws regarding disposal of used needles and syringes. Refer patients to the FDA's website at http://www.fda.gov/safesharpsdisposal for more information about safe sharps disposal, and for specific information about sharps disposal in the state that they live in.
Instruct patients not to dispose of their used sharps disposal container in their household trash unless their community guidelines permit this. Instruct patients not to recycle their used sharps disposal container.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com. For Medical Information about ABRILADA, please visit www.pfizermedinfo.com or call 1-800-438-1985.
INSTRUCTIONS FOR USE
ABRILADA (AH brill-ah-dah)
(adalimumab-afzb)
40 mg/0.8 mL
Single-dose Prefilled Pen
Injection, for subcutaneous (under the skin) use
Keep this leaflet. These instructions show step by step directions on how to prepare and give an injection.
Storage Information:
- •
- Store your ABRILADA pen in the refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Store ABRILADA pen in the original carton until use to protect from light.
- •
- Do not freeze ABRILADA. Do not use ABRILADA if frozen, even if it has been thawed.
- •
- Refrigerated ABRILADA may be used until the expiration date printed on the ABRILADA carton or pen. Do not use ABRILADA after the expiration date.
- •
- If needed, for example when you are traveling, you may also store ABRILADA at room temperature up to 86°F (30°C) for up to 30 days. Store ABRILADA in the original carton until use to protect it from light.
- •
- Throw away ABRILADA if it has been kept at room temperature and not been used within 30 days.
- •
- Record the date you first remove ABRILADA from the refrigerator in the spaces provided on the ABRILADA pen carton.
- •
- Do not store ABRILADA in extreme heat or cold.
- •
- Do not use the pen if the liquid is cloudy, discolored, or has flakes or particles in it.
Keep ABRILADA, injection supplies, and all other medicines out of the reach of children.
ABRILADA for injection comes in a disposable (throw away) single-use pen that contains a single dose of medicine.
ABRILADA for injection can be given by a patient, caregiver or healthcare provider. Do not try to inject ABRILADA yourself until you are shown the right way to give the injections and read and understand the Instructions for Use. If your healthcare provider decides that you or a caregiver may be able to give your injections of ABRILADA at home, you should receive training on the right way to prepare and inject ABRILADA. It is important that you read, understand, and follow these instructions so that you inject ABRILADA the right way.
It is important to talk to your healthcare provider to be sure you understand your ABRILADA dosing instructions. To help you remember when to inject ABRILADA, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject ABRILADA.
Step 1. Supplies you need
- •
- You will need the following supplies for each injection of ABRILADA. Find a clean, flat surface to place the supplies on.
- o
- 1 ABRILADA pen (included inside the carton)
- o
- 1 alcohol swab (included inside the carton)
- o
- 1 cotton ball or gauze pad (not included in your ABRILADA carton)
- o
- 1 puncture resistant sharps disposal container for pen disposal (not included in your ABRILADA carton). See Step 10 "Dispose of used pen" at the end of this Instructions for Use.
Step 2. Getting ready
- •
- Remove the ABRILADA carton from the refrigerator.
- •
- Make sure the name ABRILADA appears on the carton and prefilled pen label.
- •
- Take out 1 ABRILADA pen and the alcohol swab. Keep your pen out of direct sunlight. Put the original carton with any unused pens back in the refrigerator.
- •
- Do not use your pen if:
- o
- your pen or the carton containing the pen has been dropped
- o
- it has been frozen or thawed
- o
- it has been kept in direct sunlight
- o
- it appears to be damaged
- o
- the seals on a new carton are broken
- o
- it has been out of the refrigerator for more than 30 days
- o
- the expiration date has passed
- o
- the liquid is cloudy, discolored or has flakes or particles
- •
- For a more comfortable injection, you may leave your pen at room temperature for 15 to 30 minutes before your injection.
- •
- Do not warm ABRILADA in any other way (for example, do not warm it in a microwave or in hot water).
- •
- Do not shake your pen. Shaking can damage your medicine.
- •
- Wash your hands with soap and water, and dry completely.
- •
- Do not remove the cap until you are ready to inject.
- •
- Look carefully at your medicine in the window.
- •
- Make sure the medicine in the pen is clear and colorless to very light brown and free from flakes or particles.
- •
- It is normal to see one or more air bubbles in the window.
- •
- Check the expiration date on the pen label. The location of the expiration date on the pen label is shown below. Do not use the pen if the expiration date has passed.
If you have any questions about your medicine, please contact your healthcare provider or pharmacist.
- •
- Choose a different injection site each time you give an injection:
- o
- Only use the front of your thighs or your lower abdomen (belly) as shown. If you choose your abdomen, do not use the area 2 inches around your belly button (navel).
- o
- Each new injection should be given at least one inch from a site you used before.
- •
- Do not inject into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- o
- If you have psoriasis, do not inject directly into any raised, thick, red, or scaly skin patches or lesions on your skin.
- •
- Do not inject through your clothes.
- •
- Wipe the injection site with the alcohol swab.
- •
- Allow the injection site to dry. Do not fan or blow on the clean area.
- •
- Do not touch this area again before giving the injection.
- •
- Twist and pull off the cap.
- •
- Throw the cap away into a sharps disposal container. You will not need it again.
Important: Handle your pen with care to avoid an accidental needle stick injury.
Note: A needle cover stays inside the cap after cap removal.
- •
- Push your pen firmly against the skin at 90 degrees, as shown in the diagram.
Note: The needle goes into the skin as you push your pen down. You will only be able to press down the injection button in Step 7 when you are pushing down firmly enough. - •
- Keep your pen pushed against the skin until Step 9.
- •
- Press the injection button all the way down and you will hear a click. The click means the start of the injection.
- •
- Keep holding your pen firmly against the skin while the orange bar moves across the window. You will hear a 2nd click.
- •
- Wait for at least 5 more seconds after the 2nd click to make sure you get the full dose of medicine.
Note: If you cannot press down the injection button, it is because you are not pushing the pen down firmly enough at the injection site. See the Question and Answer section on the right side of this Instructions for Use for more information on what to do if the injection button does not press down.
- •
- You should see an orange bar in the window.
- •
- Do not remove your pen until you have waited at least 5 seconds after the 2nd click and until the orange bar completely fills the window.
- •
- Remove your pen from the skin.
Note: After you remove your pen from the skin, the needle will be automatically covered. - •
- If the window has not turned orange, this means you have not received a full dose. Call your healthcare provider or pharmacist right away.
- •
- Do not inject another dose.
- •
- Put your used pen in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) pens in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of heavy duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- is properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- •
- Look closely at your injection site. If there is blood, use a clean cotton ball or gauze pad to press lightly on the injection area for a few seconds.
- •
- Do not rub the injection site.
Note: Store any unused pens in the refrigerator in the original carton.
Questions and Answers
What should I do with my pen if it has been dropped?
Do not use it, even if it looks undamaged. Dispose of your pen in the same way as a used pen. You will need to use a new pen to give your injection.
Can I use my pen straight from the refrigerator?
Yes, however you may find that using the pen at room temperature reduces stinging or discomfort. If you allow your pen to reach room temperature before use, you must keep it away from direct sunlight as this can damage your medicine.
What should I do if I need to travel?
When you are traveling, you may store your pen in its carton at room temperature up to 86°F (30°C) for up to 30 days.
Is it okay to shake my pen before I use it?
No, do not shake your pen. Shaking can damage your medicine. When you check your medicine, gently tilt your pen back and forth while looking carefully into the window. It is normal to see one or more air bubbles.
Do I need to remove any air bubbles before using my pen?
No, do not attempt to remove air bubbles.
Drops of medicine have appeared at the needle tip. Is this okay?
Yes, it is normal to see a few drops of medicine at the needle tip when you remove the cap.
Can I re-insert the needle if I change my mind where I want to inject?
No, you should not re-insert the needle into your skin. If you change your mind, you will need a replacement pen if the needle has already been inserted into the skin. After the injection button has been pressed, you must not lift your pen from the skin until the injection has finished.
I pushed my pen against the skin but could not press the button down. What should I do?
Take your finger off the injection button and push your pen down more firmly against the skin. Then try pushing the button again. If this does not work, stretching the skin may make the injection site firmer, making pressing the injection button easier.
Can I pinch or stretch the skin at the injection area?
Yes, pinching or stretching the skin before injection may make the injection site firmer, making it easier to press the injection button.
Do I need to keep my finger pressed on the injection button for the whole injection?
No, you can stop pressing the button when the injection has started. However, make sure you keep holding the pen firmly against the skin. The pen will continue to deliver your medicine.
How long will the injection take?
From the time the dose begins until you hear the 2nd click, it usually takes 3 to 10 seconds. After the 2nd click, you should continue to hold your pen in place for at least 5 more seconds to make sure you give the full dose.
What should I do if I see more than a small drop of medicine on the skin after giving my injection?
Nothing this time, but for your next injection wait a little longer before removing the pen from the skin to make sure all of the medicine went into your skin.
What should I do if I have any questions about my ABRILADA pen or medicine?
Contact your healthcare provider or pharmacist.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by Pfizer Inc.
New York, NY 10001
Distributed by Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
US License No. 2001
LAB-1352-3.0
Revised: 06/2023
INSTRUCTIONS FOR USE
ABRILADA (AH brill-ah-dah)
(adalimumab-afzb)
10 mg/0.2 mL, 20 mg/0.4 mL, 40 mg/0.8 mL
Single-dose Prefilled Syringe
Injection, for subcutaneous (under the skin) use only
Keep this leaflet. These instructions show step by step directions on how to prepare and give an injection.
Storage Information:
- •
- Store your ABRILADA prefilled syringe in the refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Store ABRILADA prefilled syringe in the original carton until use to protect from light.
- •
- Do not freeze ABRILADA. Do not use ABRILADA if frozen, even if it has been thawed.
- •
- Refrigerated ABRILADA may be used until the expiration date printed on the ABRILADA carton or prefilled syringe. Do not use ABRILADA after the expiration date.
- •
- If needed, for example when you are traveling, you may also store ABRILADA at room temperature up to 86°F (30°C) for up to 30 days. Store ABRILADA in the original carton until use to protect it from light.
- •
- Throw away ABRILADA if it has been kept at room temperature and not been used within 30 days.
- •
- Record the date you first remove ABRILADA from the refrigerator in the spaces provided on the ABRILADA prefilled syringe carton.
- •
- Do not store ABRILADA in extreme heat or cold.
- •
- Do not use a prefilled syringe if the liquid is cloudy, discolored, or has flakes or particles in it.
- •
- Do not drop or crush ABRILADA. The prefilled syringe is glass.
Keep ABRILADA, injection supplies, and all other medicines out of the reach of children.
ABRILADA for injection comes in a disposable (throw away) single use prefilled syringe that contains a single dose of medicine.
ABRILADA for injection can be given by a patient, caregiver or healthcare provider. Do not try to inject ABRILADA yourself until you are shown the right way to give the injections and read and understand the Instructions for Use. If your healthcare provider decides that you or a caregiver may be able to give your injections of ABRILADA at home, you should receive training on the right way to prepare and inject ABRILADA. It is important that you read, understand, and follow these instructions so that you inject ABRILADA the right way.
It is important to talk to your healthcare provider to be sure you understand your ABRILADA dosing instructions. To help you remember when to inject ABRILADA, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject ABRILADA.
Step 1. Supplies you need
- •
- You will need the following supplies for each injection of ABRILADA. Find a clean, flat surface to place the supplies on.
- o
- 1 ABRILADA prefilled syringe in a tray inside the carton
- o
- 1 alcohol swab inside the carton
- o
- 1 cotton ball or gauze pad (not included in your ABRILADA carton)
- o
- 1 puncture resistant sharps disposal container for prefilled syringe disposal (not included in your ABRILADA carton). See Step 10 "Dispose of used syringe" at the end of this Instructions for Use.
Step 2. Getting ready
- •
- Remove ABRILADA carton from the refrigerator.
- •
- Open the carton and take out the tray containing your prefilled syringe.
- •
- Make sure the name ABRILADA appears on the dose tray and prefilled syringe label.
- •
- Check your carton and tray. Do not use if:
- o
- it has been dropped
- o
- it has been frozen or thawed
- o
- it appears to be damaged
- o
- the seals on a new carton are broken
- o
- it has been out of the refrigerator for more than 30 days
- o
- the expiration date has passed
Wash your hands with soap and water, and dry completely.
If you have any questions about your medicine, please contact your healthcare provider or pharmacist.
- •
- Peel back the paper seal on the tray.
- •
- Remove 1 prefilled syringe from the tray and put the original carton with any unused prefilled syringes back in the refrigerator.
- •
- Do not use your syringe if:
- o
- it appears to be damaged
- o
- It has been kept in direct sunlight
- o
- liquid is cloudy, discolored, or has flakes or particles
- •
- Do not shake your syringe. Shaking can damage your medicine.
- •
- For a more comfortable injection, leave the prefilled syringe at room temperature for 15 to 30 minutes before your injection.
- •
- Do not warm ABRILADA in any other way (for example, do not warm it in a microwave or in hot water).
- •
- Do not remove the needle cover from your prefilled syringe until you are ready to inject.
Always hold the prefilled syringe by the barrel to prevent damage.
- •
- Look carefully at your medicine in the window.
- •
- Make sure the medicine in the prefilled syringe is clear and colorless to very light brown and free from flakes or particles.
- •
- It is normal to see one or more air bubbles in the window.
- •
- Check the expiration date on the prefilled syringe label as shown in the figure in Step 1. Do not use the prefilled syringe if the expiration date has passed.
If you have any questions about your medicine, please contact your healthcare provider or pharmacist.
- •
- Choose a different injection site each time you give an injection.
- o
- Only use the front of your thighs or your lower abdomen (belly) as shown. If you choose your abdomen, do not use the area 2 inches around your belly button (navel).
- o
- Each new injection should be given at least one inch from a site you used before.
- •
- Do not inject into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- o
- If you have psoriasis, do not inject directly into any raised, thick, red, or scaly skin patches or lesions on your skin.
- •
- Do not inject through your clothes.
- •
- Wipe the injection site with the alcohol swab.
- •
- Allow the injection site to dry. Do not fan or blow on the clean area.
- •
- Do not touch this area again before giving the injection.
- •
- Hold the prefilled syringe by the barrel. Carefully pull the needle cover straight off and away from your body when you are ready to inject.
- •
- It is normal to see a drop of liquid at the end of the needle.
- •
- Throw the needle cover away into a sharps disposal container.
Note: Be careful when you handle your prefilled syringe to avoid an accidental needle stick injury.
- •
- Gently pinch up a fold of skin in the cleaned injection site area.
- •
- Insert the needle to its full depth into the skin, at a 45 degree angle, as shown.
- •
- After the needle is inserted, release the pinched skin.
- •
- Using slow and constant pressure, push the plunger rod all the way down until the barrel is empty.
Note: It is recommended to hold your prefilled syringe in the skin for an additional 5 seconds after the plunger has been pressed down completely to make sure you get the full dose of medicine. - •
- Pull the needle out of the skin at the same angle at which it entered.
- •
- Check that your medicine has completely emptied from your prefilled syringe. If the gray stopper is not in the position shown, you may not have injected all of your medicine. Contact your healthcare provider right away.
- •
- Never re-insert the needle.
- •
- Never put the cap back on the needle.
- •
- Put your used syringe in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of heavy duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- is properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- •
- Look closely at your injection site. If there is blood, use a clean cotton ball or gauze pad to press lightly on the injection area for a few seconds.
- •
- Do not rub the site.
Note: Store any unused syringes in the refrigerator in the original carton.
Questions and Answers
What should I do with my prefilled syringe if it has been dropped?
Do not use it if it has been dropped or the carton containing your prefilled syringe has been dropped even if it looks undamaged. Dispose of your prefilled syringe in the same way as a used prefilled syringe. You will need to use a new prefilled syringe to give your injection.
Can I use my prefilled syringe straight from the refrigerator?
Yes, however you may find that using the prefilled syringe at room temperature reduces stinging or discomfort. If you allow your prefilled syringe to reach room temperature before use, you must keep it away from direct sunlight as this can damage your medicine.
What should I do if I need to travel?
When you are traveling, you may store your prefilled syringe in its carton at room temperature up to 86°F (30°C) for up to 30 days.
Is it okay to shake my prefilled syringe before I use it?
No, do not shake your prefilled syringe. Shaking can damage your medicine. When you check your medicine, gently tilt your syringe back and forth while looking carefully into the window. It is normal to see one or more bubbles.
Do I need to remove any air bubbles before using my prefilled syringe?
No, do not attempt to remove air bubbles.
Drops of medicine have appeared at the needle tip. Is this okay?
Yes, it is normal to see a few drops of medicine at the needle tip when you remove the needle cover.
Can I re-insert the needle into my skin?
No, you should not re-insert the needle into the skin. You will need a replacement prefilled syringe if the needle has already been inserted into the skin.
How long will the injection take?
Dose delivery will take approximately 2 to 5 seconds. Remember to hold your prefilled syringe in place for at least 5 seconds after the plunger has been pushed down all the way.
What should I do if I have any questions about my prefilled syringe or medicine?
Contact your healthcare provider or pharmacist.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by Pfizer Inc.
New York, NY 10001
Distributed by Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
US License No. 2001
LAB-1353-3.0
Revised: 06/2023
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information.9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.