palonosetron HCl injection Clinical Studies
14 CLINICAL STUDIES
14.1 Prevention of Nausea and Vomiting Associated with MEC and HEC in Adults
Efficacy of a single intravenous dose of Palonosetron HCl Injection in preventing acute and delayed nausea and vomiting associated with MEC or HEC were studied in 4 trials. In these double-blind studies, complete response rates (no emetic episodes and no rescue medication) and other efficacy parameters were assessed through at least 120 hours after administration of chemotherapy. The safety and efficacy of Palonosetron HCl Injection in repeated courses of chemotherapy was also assessed.
Moderately Emetogenic Chemotherapy
Two double-blind trials (Study 1 and Study 2) involving 1,132 patients compared a single dose of Palonosetron HCl Injection with either a single-dose of ondansetron (Study 1) or dolasetron (Study 2) given 30 minutes prior to MEC, including carboplatin, cisplatin ≤ 50 mg/m², cyclophosphamide < 1,500 mg/m², doxorubicin > 25 mg/m², epirubicin, irinotecan, and methotrexate > 250 mg/m². Concomitant corticosteroids were not administered prophylactically in Study 1 and were only used by 4 to 6% of patients in Study 2. The majority of patients in these studies were women (77%), White (65%) and naïve to previous chemotherapy (54%). The mean age was 55 years.
Highly Emetogenic Chemotherapy
A double-blind, dose-ranging trial evaluated the efficacy of a single intravenous dose of Palonosetron HCl Injection from 0.3 to 90 mcg/kg (equivalent to < 0.1 mg to 6 mg fixed dose) in 161 chemotherapy-naïve adult cancer patients receiving HEC, either cisplatin ≥ 70 mg/m² or cyclophosphamide > 1,100 mg/m². Concomitant corticosteroids were not administered prophylactically. Analysis of data from this trial indicates that 0.25 mg is the lowest effective dose in preventing acute nausea and vomiting associated with HEC.
A double-blind trial involving 667 patients compared a single intravenous dose of Palonosetron HCl Injection with a single intravenous dose of ondansetron (Study 3) given 30 minutes prior to HEC, including cisplatin ≥ 60 mg/m², cyclophosphamide > 1,500 mg/m², and dacarbazine. Corticosteroids were co-administered prophylactically before chemotherapy in 67% of patients. Of the 667 patients, 51% were women, 60% White, and 59% naïve to previous chemotherapy. The mean age was 52 years.
Efficacy Results
Studies 1, 2 and 3 show that Palonosetron HCl Injection was effective in the prevention of nausea and vomiting associated with initial and repeat courses of MEC and HEC in the acute phase (0 to 24 hours) [Table 5]. Clinical superiority over other 5-HT3 receptor antagonists has not been adequately demonstrated in the acute phase. In Study 3, efficacy was greater when prophylactic corticosteroids were administered concomitantly.
Studies 1 and 2 show that Palonosetron HCl Injection was effective in the prevention of nausea and vomiting associated with initial and repeat course of MEC in the delayed phase (24 to 120 hours) [Table 6] and overall phase (0 to 120 hours) [Table 7].
| Chemotherapy | Study | Treatment Group | N* | % with Complete Response | p-value† | 97.5% Confidence Interval Palonosetron HCl Injection minus Comparator‡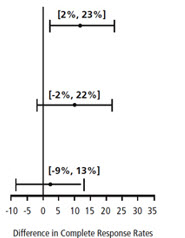 |
| Moderately Emetogenic | 1 | Palonosetron HCl Injection 0.25 mg intravenously | 189 | 81 | 0.009 | |
| Ondansetron 32 mg intravenously | 185 | 69 | ||||
| 2 | Palonosetron HCl Injection 0.25 mg intravenously | 189 | 63 | NS | ||
| Dolasetron 100 mg intravenously | 191 | 53 | ||||
| Highly Emetogenic | 3 | Palonosetron HCl Injection 0.25 mg intravenously | 223 | 59 | NS | |
| Ondansetron 32 mg intravenously | 221 | 57 | ||||
| ||||||
| Chemotherapy | Study | Treatment Group | N* | % with Complete Response | p-value† | 97.5% Confidence Interval Palonosetron HCl Injection minus Comparator‡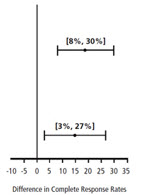 |
| Moderately Emetogenic | 1 | Palonosetron HCl Injection 0.25 mg intravenously | 189 | 74 | <0.001 | |
| Ondansetron 32 mg intravenously § | 185 | 55 | ||||
| 2 | Palonosetron HCl Injection 0.25 mg intravenously | 189 | 54 | 0.004 | ||
| Dolasetron 100 mg intravenously | 191 | 39 | ||||
| ||||||
| Chemotherapy | Study | Treatment Group | N* | % with Complete Response | p-value† | 97.5% Confidence Interval Palonosetron HCl Injection minus Comparator‡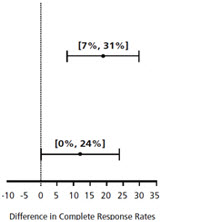 |
| Moderately Emetogenic | 1 | Palonosetron HCl Injection 0.25 mg intravenously | 189 | 69 | <0.001 | |
| Ondansetron 32 mg intravenously § | 185 | 50 | ||||
| 2 | Palonosetron HCl Injection 0.25 mg intravenously | 189 | 46 | 0.021 | ||
| Dolasetron 100 mg intravenously | 191 | 34 | ||||
14.2 Prevention of Nausea and Vomiting Associated with Emetogenic Chemotherapy, Including HEC in Pediatric Patients
One double-blind, active-controlled clinical trial was conducted in pediatric cancer patients. The total population (N = 327) had a mean age of 8.3 years (range 2 months to 16.9 years) and were 53% male; and 96% white. Patients were randomized and received a 20 mcg/kg (maximum 1.5 mg) intravenous infusion of Palonosetron HCl Injection 30 minutes prior to the start of emetogenic chemotherapy (followed by placebo infusions 4 and 8 hours after the dose of palonosetron injection) or 0.15 mg/kg of intravenous ondansetron 30 minutes prior to the start of emetogenic chemotherapy (followed by ondansetron 0.15 mg/kg infusions 4 and 8 hours after the first dose of ondansetron, with a maximum total dose of 32 mg). Emetogenic chemotherapies administered included doxorubicin, cyclophosphamide (<1,500 mg/m2), ifosfamide, cisplatin, dactinomycin, carboplatin, and daunorubicin. Adjuvant corticosteroids, including dexamethasone, were administered with chemotherapy in 55% of patients.
Complete Response in the acute phase of the first cycle of chemotherapy was defined as no vomiting, no retching, and no rescue medication in the first 24 hours after starting chemotherapy. Efficacy was based on demonstrating non-inferiority of intravenous Palonosetron HCl Injection compared to intravenous ondansetron. Non-inferiority criteria were met if the lower bound of the 97.5% confidence interval for the difference in Complete Response rates of intravenous Palonosetron HCl Injection minus intravenous ondansetron was larger than -15%. The non-inferiority margin was 15%.
Efficacy Results
As shown in Table 8, intravenous Palonosetron HCl Injection 20 mcg/kg (maximum 1.5 mg) demonstrated non-inferiority to the active comparator during the 0 to 24-hour time interval.
| Palonosetron HCl injection 20 mcg/kg intravenously (N=165) | Ondansetron 0.15 mg/kg for 3 Intravenous doses (N=162) | Difference [97.5% Confidence Interval]*: Palonosetron HCl injection minus intravenous Ondansetron Comparator |
|---|---|---|
| ||
| 59.4% | 58.6% | 0.36% [-11.7%, 12.4%] |
In patients that received Palonosetron HCl Injection at a lower dose than the recommended dose of 20 mcg/kg, non-inferiority criteria were not met.
14.3 Prevention of Postoperative Nausea and Vomiting in Adults
In a multicenter, randomized, stratified, double-blind, parallel-group, clinical trial, Palonosetron HCl Injection was compared to placebo for PONV in 546 patients undergoing abdominal and gynecological surgery. All patients received general anesthesia. The trial was conducted predominantly in the US in the out-patient setting for patients undergoing elective gynecologic or abdominal laparoscopic surgery and stratified at randomization for the following risk factors: gender, non-smoking status, history of PONV and/or motion sickness.
Patients were randomized to receive a single dose of Palonosetron HCl Injection 0.025 mg, 0.050 mg or 0.075 mg or placebo, each given intravenously immediately prior to induction of anesthesia. Antiemetic activity of palonosetron was evaluated during the 0 to 72-hour time period after surgery.
Of the 138 patients treated with palonosetron injection 0.075 mg and evaluated for efficacy, 96% were women; 66% had a history of PONV or motion sickness; 85% were non-smokers. As for race, 63% were White, 20% were Black, 15% were Hispanic, and 1% were Asian. The age of patients ranged from 21 to 74 years, with a mean age of 38 years. Three patients were greater than 65 years of age.
Co-primary efficacy measures were Complete Response (CR) defined as no emetic episode and no use of rescue medication in 0 to 24 hours and 24 to 72 hours postoperatively.
Secondary efficacy endpoints included:
- Complete Response (CR) 0 to 48 hours and 0 to 72 hours
- Complete Control (CC) defined as CR and no more than mild nausea
- Severity of nausea (none, mild, moderate, severe)
The primary hypothesis was that at least one of the three palonosetron doses were superior to placebo.
Complete Response Rates for palonosetron injection 0.075 mg and placebo in this trial are described in Table 9.
| Treatment | n/N (%) | Palonosetron injection vs Placebo | |
|---|---|---|---|
| Δ | p-value * | ||
| Δ Difference (%): palonosetron 0.075 mg minus placebo | |||
| |||
| Co-primary Endpoints | |||
| Complete Response Rate (0 to 24 hours) | |||
| Palonosetron HCl injection 0.075 mg intravenously | 59/138 (42.8%) | 16.8% | 0.004 |
| Placebo | 35/135 (25.9%) | ||
| Complete Response Rate (24 to 72 hours) | |||
| Palonosetron HCl injection 0.075 mg intravenously | 67/138 (48.6%) | 7.8% | 0.188 |
| Placebo | 55/135 (40.7%) | ||
Palonosetron HCl Injection as a single dose of 0.075 mg reduced the severity of nausea compared to placebo. Analyses of other secondary endpoints indicate that Palonosetron HCl Injection 0.075 mg was numerically better than placebo, however, statistical significance was not formally demonstrated.
A randomized, double-blind, multicenter, placebo-controlled, dose ranging study was performed to evaluate Palonosetron HCl Injection for PONV following abdominal or vaginal hysterectomy. Five intravenous doses (0.1, 0.3, 1.0, 3.0 and 30 mcg/kg) were evaluated in a total of 381 intent-to-treat patients. The primary efficacy measure was the proportion of patients with CR in the first 24 hours after recovery from surgery. The lowest effective dose was Palonosetron HCl Injection 1 mcg/kg (approximately 0.075 mg) which had a CR rate of 44% versus 19% for placebo, p=0.004 and significantly reduced the severity of nausea versus placebo, p=0.009.
Find palonosetron HCl injection medical information:
Find palonosetron HCl injection medical information:
palonosetron HCl injection Quick Finder
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 Prevention of Nausea and Vomiting Associated with MEC and HEC in Adults
Efficacy of a single intravenous dose of Palonosetron HCl Injection in preventing acute and delayed nausea and vomiting associated with MEC or HEC were studied in 4 trials. In these double-blind studies, complete response rates (no emetic episodes and no rescue medication) and other efficacy parameters were assessed through at least 120 hours after administration of chemotherapy. The safety and efficacy of Palonosetron HCl Injection in repeated courses of chemotherapy was also assessed.
Moderately Emetogenic Chemotherapy
Two double-blind trials (Study 1 and Study 2) involving 1,132 patients compared a single dose of Palonosetron HCl Injection with either a single-dose of ondansetron (Study 1) or dolasetron (Study 2) given 30 minutes prior to MEC, including carboplatin, cisplatin ≤ 50 mg/m², cyclophosphamide < 1,500 mg/m², doxorubicin > 25 mg/m², epirubicin, irinotecan, and methotrexate > 250 mg/m². Concomitant corticosteroids were not administered prophylactically in Study 1 and were only used by 4 to 6% of patients in Study 2. The majority of patients in these studies were women (77%), White (65%) and naïve to previous chemotherapy (54%). The mean age was 55 years.
Highly Emetogenic Chemotherapy
A double-blind, dose-ranging trial evaluated the efficacy of a single intravenous dose of Palonosetron HCl Injection from 0.3 to 90 mcg/kg (equivalent to < 0.1 mg to 6 mg fixed dose) in 161 chemotherapy-naïve adult cancer patients receiving HEC, either cisplatin ≥ 70 mg/m² or cyclophosphamide > 1,100 mg/m². Concomitant corticosteroids were not administered prophylactically. Analysis of data from this trial indicates that 0.25 mg is the lowest effective dose in preventing acute nausea and vomiting associated with HEC.
A double-blind trial involving 667 patients compared a single intravenous dose of Palonosetron HCl Injection with a single intravenous dose of ondansetron (Study 3) given 30 minutes prior to HEC, including cisplatin ≥ 60 mg/m², cyclophosphamide > 1,500 mg/m², and dacarbazine. Corticosteroids were co-administered prophylactically before chemotherapy in 67% of patients. Of the 667 patients, 51% were women, 60% White, and 59% naïve to previous chemotherapy. The mean age was 52 years.
Efficacy Results
Studies 1, 2 and 3 show that Palonosetron HCl Injection was effective in the prevention of nausea and vomiting associated with initial and repeat courses of MEC and HEC in the acute phase (0 to 24 hours) [Table 5]. Clinical superiority over other 5-HT3 receptor antagonists has not been adequately demonstrated in the acute phase. In Study 3, efficacy was greater when prophylactic corticosteroids were administered concomitantly.
Studies 1 and 2 show that Palonosetron HCl Injection was effective in the prevention of nausea and vomiting associated with initial and repeat course of MEC in the delayed phase (24 to 120 hours) [Table 6] and overall phase (0 to 120 hours) [Table 7].
| Chemotherapy | Study | Treatment Group | N* | % with Complete Response | p-value† | 97.5% Confidence Interval Palonosetron HCl Injection minus Comparator‡ |
| Moderately Emetogenic | 1 | Palonosetron HCl Injection 0.25 mg intravenously | 189 | 81 | 0.009 | |
| Ondansetron 32 mg intravenously | 185 | 69 | ||||
| 2 | Palonosetron HCl Injection 0.25 mg intravenously | 189 | 63 | NS | ||
| Dolasetron 100 mg intravenously | 191 | 53 | ||||
| Highly Emetogenic | 3 | Palonosetron HCl Injection 0.25 mg intravenously | 223 | 59 | NS | |
| Ondansetron 32 mg intravenously | 221 | 57 | ||||
| ||||||
| Chemotherapy | Study | Treatment Group | N* | % with Complete Response | p-value† | 97.5% Confidence Interval Palonosetron HCl Injection minus Comparator‡ |
| Moderately Emetogenic | 1 | Palonosetron HCl Injection 0.25 mg intravenously | 189 | 74 | <0.001 | |
| Ondansetron 32 mg intravenously § | 185 | 55 | ||||
| 2 | Palonosetron HCl Injection 0.25 mg intravenously | 189 | 54 | 0.004 | ||
| Dolasetron 100 mg intravenously | 191 | 39 | ||||
| ||||||
| Chemotherapy | Study | Treatment Group | N* | % with Complete Response | p-value† | 97.5% Confidence Interval Palonosetron HCl Injection minus Comparator‡ |
| Moderately Emetogenic | 1 | Palonosetron HCl Injection 0.25 mg intravenously | 189 | 69 | <0.001 | |
| Ondansetron 32 mg intravenously § | 185 | 50 | ||||
| 2 | Palonosetron HCl Injection 0.25 mg intravenously | 189 | 46 | 0.021 | ||
| Dolasetron 100 mg intravenously | 191 | 34 | ||||
14.2 Prevention of Nausea and Vomiting Associated with Emetogenic Chemotherapy, Including HEC in Pediatric Patients
One double-blind, active-controlled clinical trial was conducted in pediatric cancer patients. The total population (N = 327) had a mean age of 8.3 years (range 2 months to 16.9 years) and were 53% male; and 96% white. Patients were randomized and received a 20 mcg/kg (maximum 1.5 mg) intravenous infusion of Palonosetron HCl Injection 30 minutes prior to the start of emetogenic chemotherapy (followed by placebo infusions 4 and 8 hours after the dose of palonosetron injection) or 0.15 mg/kg of intravenous ondansetron 30 minutes prior to the start of emetogenic chemotherapy (followed by ondansetron 0.15 mg/kg infusions 4 and 8 hours after the first dose of ondansetron, with a maximum total dose of 32 mg). Emetogenic chemotherapies administered included doxorubicin, cyclophosphamide (<1,500 mg/m2), ifosfamide, cisplatin, dactinomycin, carboplatin, and daunorubicin. Adjuvant corticosteroids, including dexamethasone, were administered with chemotherapy in 55% of patients.
Complete Response in the acute phase of the first cycle of chemotherapy was defined as no vomiting, no retching, and no rescue medication in the first 24 hours after starting chemotherapy. Efficacy was based on demonstrating non-inferiority of intravenous Palonosetron HCl Injection compared to intravenous ondansetron. Non-inferiority criteria were met if the lower bound of the 97.5% confidence interval for the difference in Complete Response rates of intravenous Palonosetron HCl Injection minus intravenous ondansetron was larger than -15%. The non-inferiority margin was 15%.
Efficacy Results
As shown in Table 8, intravenous Palonosetron HCl Injection 20 mcg/kg (maximum 1.5 mg) demonstrated non-inferiority to the active comparator during the 0 to 24-hour time interval.
| Palonosetron HCl injection 20 mcg/kg intravenously (N=165) | Ondansetron 0.15 mg/kg for 3 Intravenous doses (N=162) | Difference [97.5% Confidence Interval]*: Palonosetron HCl injection minus intravenous Ondansetron Comparator |
|---|---|---|
| ||
| 59.4% | 58.6% | 0.36% [-11.7%, 12.4%] |
In patients that received Palonosetron HCl Injection at a lower dose than the recommended dose of 20 mcg/kg, non-inferiority criteria were not met.
14.3 Prevention of Postoperative Nausea and Vomiting in Adults
In a multicenter, randomized, stratified, double-blind, parallel-group, clinical trial, Palonosetron HCl Injection was compared to placebo for PONV in 546 patients undergoing abdominal and gynecological surgery. All patients received general anesthesia. The trial was conducted predominantly in the US in the out-patient setting for patients undergoing elective gynecologic or abdominal laparoscopic surgery and stratified at randomization for the following risk factors: gender, non-smoking status, history of PONV and/or motion sickness.
Patients were randomized to receive a single dose of Palonosetron HCl Injection 0.025 mg, 0.050 mg or 0.075 mg or placebo, each given intravenously immediately prior to induction of anesthesia. Antiemetic activity of palonosetron was evaluated during the 0 to 72-hour time period after surgery.
Of the 138 patients treated with palonosetron injection 0.075 mg and evaluated for efficacy, 96% were women; 66% had a history of PONV or motion sickness; 85% were non-smokers. As for race, 63% were White, 20% were Black, 15% were Hispanic, and 1% were Asian. The age of patients ranged from 21 to 74 years, with a mean age of 38 years. Three patients were greater than 65 years of age.
Co-primary efficacy measures were Complete Response (CR) defined as no emetic episode and no use of rescue medication in 0 to 24 hours and 24 to 72 hours postoperatively.
Secondary efficacy endpoints included:
- Complete Response (CR) 0 to 48 hours and 0 to 72 hours
- Complete Control (CC) defined as CR and no more than mild nausea
- Severity of nausea (none, mild, moderate, severe)
The primary hypothesis was that at least one of the three palonosetron doses were superior to placebo.
Complete Response Rates for palonosetron injection 0.075 mg and placebo in this trial are described in Table 9.
| Treatment | n/N (%) | Palonosetron injection vs Placebo | |
|---|---|---|---|
| Δ | p-value * | ||
| Δ Difference (%): palonosetron 0.075 mg minus placebo | |||
| |||
| Co-primary Endpoints | |||
| Complete Response Rate (0 to 24 hours) | |||
| Palonosetron HCl injection 0.075 mg intravenously | 59/138 (42.8%) | 16.8% | 0.004 |
| Placebo | 35/135 (25.9%) | ||
| Complete Response Rate (24 to 72 hours) | |||
| Palonosetron HCl injection 0.075 mg intravenously | 67/138 (48.6%) | 7.8% | 0.188 |
| Placebo | 55/135 (40.7%) | ||
Palonosetron HCl Injection as a single dose of 0.075 mg reduced the severity of nausea compared to placebo. Analyses of other secondary endpoints indicate that Palonosetron HCl Injection 0.075 mg was numerically better than placebo, however, statistical significance was not formally demonstrated.
A randomized, double-blind, multicenter, placebo-controlled, dose ranging study was performed to evaluate Palonosetron HCl Injection for PONV following abdominal or vaginal hysterectomy. Five intravenous doses (0.1, 0.3, 1.0, 3.0 and 30 mcg/kg) were evaluated in a total of 381 intent-to-treat patients. The primary efficacy measure was the proportion of patients with CR in the first 24 hours after recovery from surgery. The lowest effective dose was Palonosetron HCl Injection 1 mcg/kg (approximately 0.075 mg) which had a CR rate of 44% versus 19% for placebo, p=0.004 and significantly reduced the severity of nausea versus placebo, p=0.009.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.