MEDROL® TABLETS
(methylprednisolone TABLETS)
Find MEDROL® TABLETS medical information:
Find MEDROL® TABLETS medical information:
MEDROL® TABLETS Quick Finder
Indications and Usage
INDICATIONS AND USAGE
MEDROL Tablets are indicated in the following conditions:
1. Endocrine Disorders
Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first choice; synthetic analogs may be used in conjunction with mineralocorticoids where applicable; in infancy mineralocorticoid supplementation is of particular importance).
Congenital adrenal hyperplasia
Nonsuppurative thyroiditis
Hypercalcemia associated with cancer
2. Rheumatic Disorders
As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in:
Rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy)
Ankylosing spondylitis
Acute and subacute bursitis
Synovitis of osteoarthritis
Acute nonspecific tenosynovitis
Post-traumatic osteoarthritis
Psoriatic arthritis
Epicondylitis
Acute gouty arthritis
3. Collagen Diseases
During an exacerbation or as maintenance therapy in selected cases of:
Systemic lupus erythematosus
Systemic dermatomyositis (polymyositis)
Acute rheumatic carditis
4. Dermatologic Diseases
Bullous dermatitis herpetiformis
Severe erythema multiforme
(Stevens-Johnson syndrome)
Severe seborrheic dermatitis
Exfoliative dermatitis
Mycosis fungoides
Pemphigus
Severe psoriasis
5. Allergic States
Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment:
Seasonal or perennial allergic rhinitis
Drug hypersensitivity reactions
Serum sickness
Contact dermatitis
Bronchial asthma
Atopic dermatitis
6. Ophthalmic Diseases
Severe acute and chronic allergic and inflammatory processes involving the eye and its adnexa such as:
Allergic corneal marginal ulcers
Herpes zoster ophthalmicus
Anterior segment inflammation
Diffuse posterior uveitis and choroiditis
Sympathetic ophthalmia
Keratitis
Optic neuritis
Allergic conjunctivitis
Chorioretinitis
Iritis and iridocyclitis
7. Respiratory Diseases
Symptomatic sarcoidosis
Berylliosis
Loeffler's syndrome not manageable by other means
Fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapy
Aspiration pneumonitis
8. Hematologic Disorders
Idiopathic thrombocytopenic purpura in adults
Secondary thrombocytopenia in adults
Acquired (autoimmune) hemolytic anemia
Erythroblastopenia (RBC anemia)
Congenital (erythroid) hypoplastic anemia
9. Neoplastic Diseases
For palliative management of:
Leukemias and lymphomas in adults
Acute leukemia of childhood
10. Edematous States
To induce a diuresis or remission of proteinuria in the nephrotic syndrome, without uremia, of the idiopathic type or that due to lupus erythematosus.
Dosage and Administration
DOSAGE AND ADMINISTRATION
The initial dosage of MEDROL Tablets may vary from 4 mg to 48 mg of methylprednisolone per day depending on the specific disease entity being treated. In situations of less severity lower doses will generally suffice while in selected patients higher initial doses may be required. The initial dosage should be maintained or adjusted until a satisfactory response is noted. If after a reasonable period of time there is a lack of satisfactory clinical response, MEDROL should be discontinued and the patient transferred to other appropriate therapy.
IT SHOULD BE EMPHASIZED THAT DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE UNDER TREATMENT AND THE RESPONSE OF THE PATIENT. After a favorable response is noted, the proper maintenance dosage should be determined by decreasing the initial drug dosage in small decrements at appropriate time intervals until the lowest dosage which will maintain an adequate clinical response is reached. It should be kept in mind that constant monitoring is needed in regard to drug dosage. Included in the situations which may make dosage adjustments necessary are changes in clinical status secondary to remissions or exacerbations in the disease process, the patient's individual drug responsiveness, and the effect of patient exposure to stressful situations not directly related to the disease entity under treatment; in this latter situation it may be necessary to increase the dosage of MEDROL for a period of time consistent with the patient's condition. If after long-term therapy the drug is to be stopped, it is recommended that it be withdrawn gradually rather than abruptly.
Multiple Sclerosis
In treatment of acute exacerbations of multiple sclerosis daily doses of 200 mg of prednisolone for a week followed by 80 mg every other day for 1 month have been shown to be effective (4 mg of methylprednisolone is equivalent to 5 mg of prednisolone).
ADT (Alternate Day Therapy)
Alternate day therapy is a corticosteroid dosing regimen in which twice the usual daily dose of corticoid is administered every other morning. The purpose of this mode of therapy is to provide the patient requiring long-term pharmacologic dose treatment with the beneficial effects of corticoids while minimizing certain undesirable effects, including pituitary-adrenal suppression, the Cushingoid state, corticoid withdrawal symptoms, and growth suppression in children.
The rationale for this treatment schedule is based on two major premises: (a) the anti-inflammatory or therapeutic effect of corticoids persists longer than their physical presence and metabolic effects and (b) administration of the corticosteroid every other morning allows for reestablishment of more nearly normal hypothalamic-pituitary-adrenal (HPA) activity on the off-steroid day.
A brief review of the HPA physiology may be helpful in understanding this rationale. Acting primarily through the hypothalamus a fall in free cortisol stimulates the pituitary gland to produce increasing amounts of corticotropin (ACTH) while a rise in free cortisol inhibits ACTH secretion. Normally the HPA system is characterized by diurnal (circadian) rhythm. Serum levels of ACTH rise from a low point about 10 pm to a peak level about 6 am. Increasing levels of ACTH stimulate adrenal cortical activity resulting in a rise in plasma cortisol with maximal levels occurring between 2 am and 8 am. This rise in cortisol dampens ACTH production and in turn adrenal cortical activity. There is a gradual fall in plasma corticoids during the day with lowest levels occurring about midnight.
The diurnal rhythm of the HPA axis is lost in Cushing's disease, a syndrome of adrenal cortical hyperfunction characterized by obesity with centripetal fat distribution, thinning of the skin with easy bruisability, muscle wasting with weakness, hypertension, latent diabetes, osteoporosis, electrolyte imbalance, etc. The same clinical findings of hyperadrenocorticism may be noted during long-term pharmacologic dose corticoid therapy administered in conventional daily divided doses. It would appear, then, that a disturbance in the diurnal cycle with maintenance of elevated corticoid values during the night may play a significant role in the development of undesirable corticoid effects. Escape from these constantly elevated plasma levels for even short periods of time may be instrumental in protecting against undesirable pharmacologic effects.
During conventional pharmacologic dose corticosteroid therapy, ACTH production is inhibited with subsequent suppression of cortisol production by the adrenal cortex. Recovery time for normal HPA activity is variable depending upon the dose and duration of treatment. During this time the patient is vulnerable to any stressful situation. Although it has been shown that there is considerably less adrenal suppression following a single morning dose of prednisolone (10 mg) as opposed to a quarter of that dose administered every six hours, there is evidence that some suppressive effect on adrenal activity may be carried over into the following day when pharmacologic doses are used. Further, it has been shown that a single dose of certain corticosteroids will produce adrenal cortical suppression for two or more days. Other corticoids, including methylprednisolone, hydrocortisone, prednisone, and prednisolone, are considered to be short acting (producing adrenal cortical suppression for 1¼ to 1½ days following a single dose) and thus are recommended for alternate day therapy.
The following should be kept in mind when considering alternate day therapy:
- 1.
- Basic principles and indications for corticosteroid therapy should apply. The benefits of ADT should not encourage the indiscriminate use of steroids.
- 2.
- ADT is a therapeutic technique primarily designed for patients in whom long-term pharmacologic corticoid therapy is anticipated.
- 3.
- In less severe disease processes in which corticoid therapy is indicated, it may be possible to initiate treatment with ADT. More severe disease states usually will require daily divided high dose therapy for initial control of the disease process. The initial suppressive dose level should be continued until satisfactory clinical response is obtained, usually four to ten days in the case of many allergic and collagen diseases. It is important to keep the period of initial suppressive dose as brief as possible particularly when subsequent use of alternate day therapy is intended.
Once control has been established, two courses are available: (a) change to ADT and then gradually reduce the amount of corticoid given every other day or (b) following control of the disease process reduce the daily dose of corticoid to the lowest effective level as rapidly as possible and then change over to an alternate day schedule. Theoretically, course (a) may be preferable. - 4.
- Because of the advantages of ADT, it may be desirable to try patients on this form of therapy who have been on daily corticoids for long periods of time (eg, patients with rheumatoid arthritis). Since these patients may already have a suppressed HPA axis, establishing them on ADT may be difficult and not always successful. However, it is recommended that regular attempts be made to change them over. It may be helpful to triple or even quadruple the daily maintenance dose and administer this every other day rather than just doubling the daily dose if difficulty is encountered. Once the patient is again controlled, an attempt should be made to reduce this dose to a minimum.
- 5.
- As indicated above, certain corticosteroids, because of their prolonged suppressive effect on adrenal activity, are not recommended for alternate day therapy (eg, dexamethasone and betamethasone).
- 6.
- The maximal activity of the adrenal cortex is between 2 am and 8 am, and it is minimal between 4 pm and midnight. Exogenous corticosteroids suppress adrenocortical activity the least, when given at the time of maximal activity (am).
- 7.
- In using ADT it is important, as in all therapeutic situations to individualize and tailor the therapy to each patient. Complete control of symptoms will not be possible in all patients. An explanation of the benefits of ADT will help the patient to understand and tolerate the possible flare-up in symptoms which may occur in the latter part of the offsteroid day. Other symptomatic therapy may be added or increased at this time if needed.
- 8.
- In the event of an acute flare-up of the disease process, it may be necessary to return to a full suppressive daily divided corticoid dose for control. Once control is again established alternate day therapy may be reinstituted.
- 9.
- Although many of the undesirable features of corticosteroid therapy can be minimized by ADT, as in any therapeutic situation, the physician must carefully weigh the benefit-risk ratio for each patient in whom corticoid therapy is being considered.
Contraindications
Warnings and Precautions
WARNINGS
In patients on corticosteroid therapy subjected to unusual stress, increased dosage of rapidly acting corticosteroids before, during, and after the stressful situation is indicated.
Immunosuppression and Increased Risk of Infection
Corticosteroids, including MEDROL, suppress the immune system and increase the risk of infection with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic pathogens. Corticosteroids can:
- •
- Reduce resistance to new infections
- •
- Exacerbate existing infections
- •
- Increase the risk of disseminated infections
- •
- Increase the risk of reactivation or exacerbation of latent infections
- •
- Mask some signs of infection
Corticosteroid-associated infections can be mild but can be severe and at times fatal. The rate of infectious complications increases with increasing corticosteroid dosages.
Monitor for the development of infection and consider MEDROL withdrawal or dosage reduction as needed.
Tuberculosis
If MEDROL is used to treat a condition in patients with latent tuberculosis or tuberculin reactivity, reactivation of tuberculosis may occur. Closely monitor such patients for reactivation. During prolonged MEDROL therapy, patients with latent tuberculosis or tuberculin reactivity should receive chemoprophylaxis.
Varicella Zoster and Measles Viral Infections
Varicella and measles can have a serious or even fatal course in non-immune patients taking corticosteroids, including MEDROL. In corticosteroid-treated patients who have not had these diseases or are non-immune, particular care should be taken to avoid exposure to varicella and measles:
- •
- If a MEDROL-treated patient is exposed to varicella, prophylaxis with varicella zoster immune globulin may be indicated. If varicella develops, treatment with antiviral agents may be considered.
- •
- If a MEDROL-treated patient is exposed to measles, prophylaxis with immunoglobulin may be indicated.
Hepatitis B Virus Reactivation
Hepatitis B virus reactivation can occur in patients who are hepatitis B carriers treated with immunosuppressive dosages of corticosteroids, including MEDROL. Reactivation can also occur infrequently in corticosteroid-treated patients who appear to have resolved hepatitis B infection.
Screen patients for hepatitis B infection before initiating immunosuppressive (e.g., prolonged) treatment with MEDROL. For patients who show evidence of hepatitis B infection, recommend consultation with physicians with expertise in managing hepatitis B regarding monitoring and consideration for hepatitis B antiviral therapy.
Fungal Infections
Corticosteroids, including MEDROL, may exacerbate systemic fungal infections; therefore, avoid MEDROL use in the presence of such infections unless MEDROL is needed to control drug reactions. For patients on chronic MEDROL therapy who develop systemic fungal infections, MEDROL withdrawal or dosage reduction is recommended.
Amebiasis
Corticosteroids, including MEDROL, may activate latent amebiasis. Therefore, it is recommended that latent amebiasis or active amebiasis be ruled out before initiating MEDROL in patients who have spent time in the tropics or patients with unexplained diarrhea.
Strongyloides Infestation
Corticosteroids, including MEDROL, should be used with great care in patients with known or suspected Strongyloides (threadworm) infestation. In such patients, corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia.
Cerebral Malaria
Avoid corticosteroids, including MEDROL, in patients with cerebral malaria.
Ophthalmic Effects
Prolonged use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to fungi or viruses.
Kaposi’s Sarcoma
Kaposi’s sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement of Kaposi’s sarcoma.
Hypertension, Volume Overload, and Hypokalemia
Average and large doses of hydrocortisone or cortisone can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretion.
Vaccination
Administration of live or live, attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of corticosteroids. Killed or inactivated vaccines may be administered to patients receiving immunosuppressive doses of corticosteroids; however, the response to such vaccines may be diminished. Indicated immunization procedures may be undertaken in patients receiving nonimmunosuppressive doses of corticosteroids.
Usage in Pregnancy
Since adequate human reproduction studies have not been done with corticosteroids, the use of these drugs in pregnancy, nursing mothers or women of child-bearing potential requires that the possible benefits of the drug be weighed against the potential hazards to the mother and embryo or fetus. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy, should be carefully observed for signs of hypoadrenalism.
PRECAUTIONS
General Precautions
Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. Since mineralocorticoid secretion may be impaired, salt and/or a mineralocorticoid should be administered concurrently.
There is an enhanced effect of corticosteroids on patients with hypothyroidism and in those with cirrhosis.
Corticosteroids should be used cautiously in patients with ocular herpes simplex because of possible corneal perforation.
The lowest possible dose of corticosteroid should be used to control the condition under treatment, and when reduction in dosage is possible, the reduction should be gradual.
Psychic derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids.
Caution is required in patients with systemic sclerosis because an increased incidence of scleroderma renal crisis has been observed with corticosteroids, including methylprednisolone.
Steroids should be used with caution in nonspecific ulcerative colitis, if there is a probability of impending perforation, abscess or other pyogenic infection; diverticulitis; fresh intestinal anastomoses; active or latent peptic ulcer; renal insufficiency; hypertension; osteoporosis; and myasthenia gravis.
Growth and development of infants and children on prolonged corticosteroid therapy should be carefully observed.
Although controlled clinical trials have shown corticosteroids to be effective in speeding the resolution of acute exacerbations of multiple sclerosis, they do not show that corticosteroids affect the ultimate outcome or natural history of the disease. The studies do show that relatively high doses of corticosteroids are necessary to demonstrate a significant effect. (See DOSAGE AND ADMINISTRATION.)
Since complications of treatment with glucocorticoids are dependent on the size of the dose and the duration of treatment, a risk/benefit decision must be made in each individual case as to dose and duration of treatment and as to whether daily or intermittent therapy should be used.
In post marketing experience, tumor lysis syndrome (TLS) has been reported in patients with malignancies, including hematological malignancies and solid tumors, following the use of systemic corticosteroids alone or in combination with other chemotherapeutic agents. Patients at high risk of TLS, such as patients with tumors that have a high proliferative rate, high tumor burden and high sensitivity to cytotoxic agents, should be monitored closely and appropriate precautions should be taken.
DRUG INTERACTIONS
The pharmacokinetic interactions listed below are potentially clinically important. Mutual inhibition of metabolism occurs with concurrent use of cyclosporin and methylprednisolone; therefore, it is possible that adverse events associated with the individual use of either drug may be more apt to occur. Convulsions have been reported with concurrent use of methylprednisolone and cyclosporin. Drugs that induce hepatic enzymes such as phenobarbital, phenytoin and rifampin may increase the clearance of methylprednisolone and may require increases in methylprednisolone dose to achieve the desired response. Drugs such as troleandomycin and ketoconazole may inhibit the metabolism of methylprednisolone and thus decrease its clearance. Therefore, the dose of methylprednisolone should be titrated to avoid steroid toxicity.
Methylprednisolone may increase the clearance of chronic high dose aspirin. This could lead to decreased salicylate serum levels or increase the risk of salicylate toxicity when methylprednisolone is withdrawn. Aspirin should be used cautiously in conjunction with corticosteroids in patients suffering from hypoprothrombinemia.
The effect of methylprednisolone on oral anticoagulants is variable. There are reports of enhanced as well as diminished effects of anticoagulant when given concurrently with corticosteroids. Therefore, coagulation indices should be monitored to maintain the desired anticoagulant effect.
Adverse Reactions
ADVERSE REACTIONS
Fluid and Electrolyte Disturbances
- •
- Sodium retention
- •
- Congestive heart failure in susceptible patients
- •
- Hypertension
- •
- Fluid retention
- •
- Potassium loss
- •
- Hypokalemic alkalosis
Musculoskeletal
- •
- Muscle weakness
- •
- Loss of muscle mass
- •
- Steroid myopathy
- •
- Osteoporosis
- •
- Tendon rupture, particularly of the Achilles tendon
- •
- Vertebral compression fractures
- •
- Aseptic necrosis of femoral and humeral heads
- •
- Pathologic fracture of long bones
Gastrointestinal
- •
- Peptic ulcer with possible perforation and hemorrhage
- •
- Pancreatitis
- •
- Abdominal distention
- •
- Ulcerative esophagitis
Increases in alanine transaminase (ALT, SGPT), aspartate transaminase (AST, SGOT), and alkaline phosphatase have been observed following corticosteroid treatment. These changes are usually small, not associated with any clinical syndrome and are reversible upon discontinuation.
Dermatologic
- •
- Impaired wound healing
- •
- Petechiae and ecchymoses
- •
- May suppress reactions to skin tests
- •
- Thin fragile skin
- •
- Facial erythema
- •
- Increased sweating
Neurological
- •
- Increased intracranial pressure with papilledema (pseudo-tumor cerebri) usually after treatment
- •
- Convulsions
- •
- Vertigo
- •
- Headache
Endocrine
- •
- Development of Cushingoid state
- •
- Suppression of growth in children
- •
- Secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery or illness
- •
- Menstrual irregularities
- •
- Decreased carbohydrate tolerance
- •
- Manifestations of latent diabetes mellitus
- •
- Increased requirements of insulin or oral hypoglycemic agents in diabetics
Ophthalmic
- •
- Posterior subcapsular cataracts
- •
- Increased intraocular pressure
- •
- Glaucoma
- •
- Exophthalmos
Metabolic
- •
- Negative nitrogen balance due to protein catabolism
The following additional reactions have been reported following oral as well as parenteral therapy: Urticaria and other allergic, anaphylactic or hypersensitivity reactions.
Description
DESCRIPTION
MEDROL Tablets contain methylprednisolone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. Methylprednisolone occurs as a white to practically white, odorless, crystalline powder. It is sparingly soluble in alcohol, in dioxane, and in methanol, slightly soluble in acetone, and in chloroform, and very slightly soluble in ether. It is practically insoluble in water.
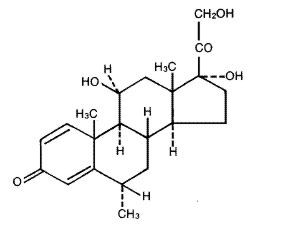
The chemical name for methylprednisolone is pregna-1,4-diene-3,20-dione, 11,17,21-trihydroxy-6-methyl-, (6α,11β)-and the molecular weight is 374.48. The structural formula is represented below:
Each MEDROL Tablet for oral administration contains 2 mg, 4 mg, 8 mg, 16 mg or 32 mg of methylprednisolone.
Inactive ingredients:
2 mg | 4 mg and 8 mg |
Calcium Stearate | Calcium Stearate |
Corn Starch | Corn Starch |
Erythrosine Sodium | Lactose |
Lactose | Sucrose |
Mineral Oil | |
Sorbic Acid | |
Sucrose | |
16 mg and 32 mg | |
Calcium Stearate | |
Corn Starch | |
Lactose | |
Mineral Oil | |
Sucrose | |
ACTIONS
Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs are primarily used for their potent anti-inflammatory effects in disorders of many organ systems.
Glucocorticoids cause profound and varied metabolic effects. In addition, they modify the body's immune responses to diverse stimuli.
References
REFERENCES
1 Fekety R. Infections associated with corticosteroids and immunosuppressive therapy. In: Gorbach SL, Bartlett JG, Blacklow NR, eds. Infectious Diseases. Philadelphia: WBSaunders Company 1992:1050–1.
2 Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticoids. Rev Infect Dis 1989:11(6):954–63.
How Supplied/Storage and Handling
HOW SUPPLIED
MEDROL Tablets are available in the following strengths and package sizes:
2 mg (white, elliptical, scored, imprinted MEDROL 2)
Bottles of 100 NDC 0009-0020-01
4 mg (white, elliptical, scored, imprinted MEDROL 4)
Bottles of 100 NDC 0009-0056-02
DOSEPAK™ Unit of Use (21 tablets) NDC 0009-0056-04
8 mg (white, elliptical, scored, imprinted MEDROL 8)
Bottles of 25 NDC 0009-0022-01
16 mg (white, elliptical, scored, imprinted MEDROL 16)
Bottles of 50 NDC 0009-0073-01
32 mg (white, elliptical, scored, imprinted MEDROL 32)
Bottles of 25 NDC 0009-0176-01
Other
This product's label may have been updated. For current full prescribing information, please visit www.pfizer.com.
LAB-0157-10.0
Revised March 2024
Resources
Didn’t find what you were looking for?
Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this product, click the link below to submit your information: Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer product, please call Pfizer Medical Information at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800)-332-1088.