lidocaine hydrochloride injection, USP ANSYR, ABBOJECT Description
DESCRIPTION
Lidocaine Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of an antiarrhythmic agent administered intravenously by either direct injection or continuous infusion. It is available in various concentrations with the following characteristics:
| Volume (Total Lidocaine HCl) | Lidocaine Hydrochloride (mg/mL) | pH | |
|---|---|---|---|
For Direct Intravenous Injection: | |||
5 mL (100 mg) | 20 | 5.0 to 7.0 | |
Single-dose: | |||
5 mL (50 mg) | 10 | 5.0 to 7.0 | |
May contain sodium hydroxide and/or hydrochloric acid for pH adjustment. Injections containing 10 mg/mL (1%) contain sodium chloride 7 mg and injections containing 20 mg/mL (2%) lidocaine hydrochloride contain sodium chloride 6 mg to adjust tonicity. Single-dose solutions contain no preservative and unused portions must be discarded after use.
Lidocaine Hydrochloride, USP is chemically designated 2-(Diethylamino)-2',6'-acetoxylidide monohydrochloride monohydrate, a white powder freely soluble in water. The molecular formula is C14H22N2O • HCl • H2O. The molecular weight is 288.82. It has the following structural formula:
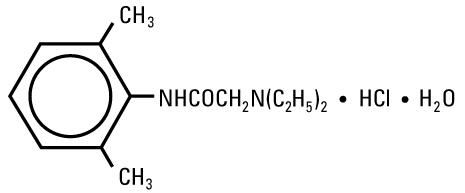
The semi-rigid vial used for the plastic vials is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The container requires no vapor barrier to maintain the proper drug concentration.
The plastic syringe is molded from a specially formulated polypropylene. Water permeates from inside the container at an extremely slow rate which will have an insignificant effect on solution concentration over the expected shelf life. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the syringe material.
Find lidocaine hydrochloride injection, USP ANSYR, ABBOJECT medical information:
Find lidocaine hydrochloride injection, USP ANSYR, ABBOJECT medical information:
lidocaine hydrochloride injection, USP ANSYR, ABBOJECT Quick Finder
Health Professional Information
Description
DESCRIPTION
Lidocaine Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of an antiarrhythmic agent administered intravenously by either direct injection or continuous infusion. It is available in various concentrations with the following characteristics:
| Volume (Total Lidocaine HCl) | Lidocaine Hydrochloride (mg/mL) | pH | |
|---|---|---|---|
For Direct Intravenous Injection: | |||
5 mL (100 mg) | 20 | 5.0 to 7.0 | |
Single-dose: | |||
5 mL (50 mg) | 10 | 5.0 to 7.0 | |
May contain sodium hydroxide and/or hydrochloric acid for pH adjustment. Injections containing 10 mg/mL (1%) contain sodium chloride 7 mg and injections containing 20 mg/mL (2%) lidocaine hydrochloride contain sodium chloride 6 mg to adjust tonicity. Single-dose solutions contain no preservative and unused portions must be discarded after use.
Lidocaine Hydrochloride, USP is chemically designated 2-(Diethylamino)-2',6'-acetoxylidide monohydrochloride monohydrate, a white powder freely soluble in water. The molecular formula is C14H22N2O • HCl • H2O. The molecular weight is 288.82. It has the following structural formula:
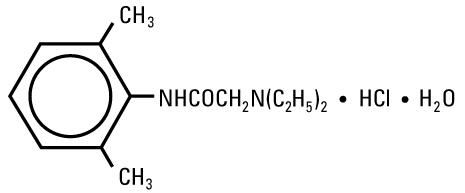
The semi-rigid vial used for the plastic vials is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The container requires no vapor barrier to maintain the proper drug concentration.
The plastic syringe is molded from a specially formulated polypropylene. Water permeates from inside the container at an extremely slow rate which will have an insignificant effect on solution concentration over the expected shelf life. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the syringe material.
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.