IBRANCE® tablets Clinical Studies
(palbociclib)
14 CLINICAL STUDIES
PALOMA-2: IBRANCE plus Letrozole
Patients with ER-positive, HER2-negative advanced or metastatic breast cancer for initial endocrine based therapy
PALOMA-2 was an international, randomized, double-blind, parallel-group, multicenter study of IBRANCE plus letrozole versus placebo plus letrozole conducted in postmenopausal women with ER-positive, HER2-negative advanced breast cancer who had not received previous systemic treatment for their advanced disease. A total of 666 patients were randomized 2:1 to IBRANCE plus letrozole or placebo plus letrozole. Randomization was stratified by disease site (visceral versus non-visceral), disease-free interval (de novo metastatic versus ≤12 months from the end of adjuvant treatment to disease recurrence versus >12 months from the end of adjuvant treatment to disease recurrence), and nature of prior (neo)adjuvant anticancer therapies (prior hormonal therapies versus no prior hormonal therapy). IBRANCE was given orally at a dose of 125 mg daily for 21 consecutive days followed by 7 days off treatment. Patients received study treatment until objective disease progression, symptomatic deterioration, unacceptable toxicity, death, or withdrawal of consent, whichever occurred first. The major efficacy outcome of the study was investigator-assessed progression-free survival (PFS) evaluated according to Response Evaluation Criteria in Solid Tumors Version 1.1 (RECIST). Additional efficacy outcome measures were confirmed overall response rate (ORR) as assessed by the investigator according to RECIST Version 1.1 and overall survival (OS).
Patients enrolled in this study had a median age of 62 years (range 28 to 89). The majority of patients were White (78%), and most patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1 (98%). Forty-eight percent of patients had received chemotherapy and 56% had received antihormonal therapy in the neoadjuvant or adjuvant setting prior to their diagnosis of advanced breast cancer. Thirty-seven percent of patients had no prior systemic therapy in the neoadjuvant or adjuvant setting. The majority of patients (97%) had metastatic disease. Twenty-three percent of patients had bone only disease, and 49% of patients had visceral disease.
Major efficacy results from PALOMA-2 are summarized in Table 8 and Figure 1. Consistent results were observed across patient subgroups of disease-free interval (DFI), disease site, and prior therapy. The treatment effect of the combination on PFS was also supported by an independent review of radiographs. Based on the prespecified final OS analysis conducted after 435 events, OS was not statistically significant.
| IBRANCE plus Letrozole | Placebo plus Letrozole | |
|---|---|---|
| CI=confidence interval; ITT=Intent-to-Treat; N=number of patients; NE=not estimable; OS=Overall survival; PFS=Progression‑free survival. | ||
Progression-free survival for ITT (investigator assessment) | N=444 | N=222 |
Number of PFS events (%) | 194 (43.7) | 137 (61.7) |
Median progression-free survival | 24.8 (22.1, NE) | 14.5 (12.9, 17.1) |
Hazard ratio (95% CI) and p-value | ||
Objective Response for patients with measurable disease (investigator assessment) | N=338 | N=171 |
Objective response rate‡ (%, 95% CI) | 55.3 (49.9, 60.7) | 44.4 (36.9, 52.2) |
Overall survival for ITT | N=444 | N=222 |
Number of OS events (%) | 287 (64.6) | 148 (66.7) |
Median OS (months, 95% CI) | 53.8 (49.8, 59.2) | 49.8 (42.3, 56.4) |
Hazard ratio (95% CI) and p‑value | ||
Figure 1. Kaplan-Meier Plot of Progression-Free Survival – PALOMA-2 (Investigator Assessment, Intent-to-Treat Population) | ||
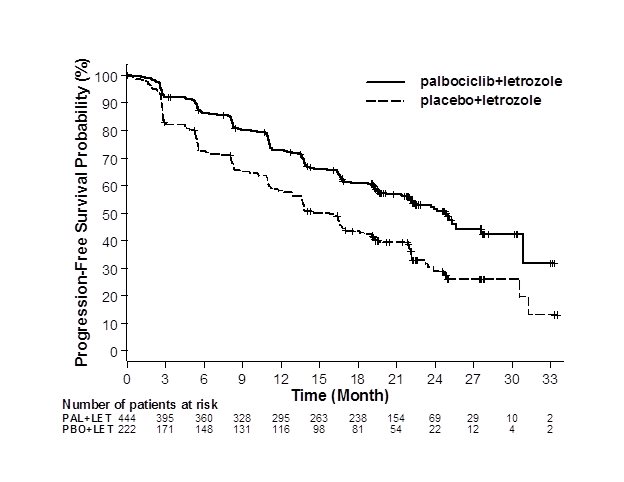 | ||
LET=letrozole; PAL=palbociclib; PBO=placebo. | ||
PALOMA-3: IBRANCE plus Fulvestrant
Patients with HR-positive, HER2-negative advanced or metastatic breast cancer who have had disease progression on or after prior adjuvant or metastatic endocrine therapy
PALOMA-3 was an international, randomized, double-blind, parallel group, multicenter study of IBRANCE plus fulvestrant versus placebo plus fulvestrant conducted in women with HR-positive, HER2-negative advanced breast cancer, regardless of their menopausal status, whose disease progressed on or after prior endocrine therapy. A total of 521 pre/postmenopausal women were randomized 2:1 to IBRANCE plus fulvestrant or placebo plus fulvestrant and stratified by documented sensitivity to prior hormonal therapy, menopausal status at study entry (pre/peri versus postmenopausal), and presence of visceral metastases. IBRANCE was given orally at a dose of 125 mg daily for 21 consecutive days followed by 7 days off treatment. Pre/perimenopausal women were enrolled in the study and received the LHRH agonist goserelin for at least 4 weeks prior to and for the duration of PALOMA-3. Patients continued to receive assigned treatment until objective disease progression, symptomatic deterioration, unacceptable toxicity, death, or withdrawal of consent, whichever occurred first. The major efficacy outcome of the study was investigator-assessed PFS evaluated according to RECIST 1.1.
Patients enrolled in this study had a median age of 57 years (range 29 to 88). The majority of patients on study were White (74%), all patients had an ECOG PS of 0 or 1, and 80% were postmenopausal. All patients had received prior systemic therapy, and 75% of patients had received a previous chemotherapy regimen. Twenty-five percent of patients had received no prior therapy in the metastatic disease setting, 60% had visceral metastases, and 23% had bone only disease.
The results from the investigator-assessed PFS and final OS from PALOMA-3 are summarized in Table 9. The relevant Kaplan-Meier plots are shown in Figures 2 and 3, respectively. Consistent PFS results were observed across patient subgroups of disease site, sensitivity to prior hormonal therapy, and menopausal status. After a median follow-up time of 45 months, the final OS results were not statistically significant.
| IBRANCE plus Fulvestrant | Placebo plus Fulvestrant | |
|---|---|---|
| CI=confidence interval; ITT=Intent-to-Treat; N=number of patients; OS=overall survival; PFS=progression-free survival. | ||
Progression-free survival for ITT | N=347 | N=174 |
Number of PFS events (%) | 145 (41.8) | 114 (65.5) |
Median PFS (months, 95% CI) | 9.5 (9.2, 11.0) | 4.6 (3.5, 5.6) |
Hazard ratio (95% CI) and p-value | 0.461 (0.360, 0.591), p<0.0001 | |
Objective Response for patients with measurable disease | N=267 | N=138 |
Objective response rate* (%, 95% CI) | 24.6 (19.6, 30.2) | 10.9 (6.2, 17.3) |
Overall survival for ITT | N=347 | N=174 |
Number of OS events (%) | 201 (57.9) | 109 (62.6) |
Median OS (months, 95% CI) | 34.9 (28.8, 40.0) | 28.0 (23.6, 34.6) |
Hazard ratio (95% CI) and p-value | ||
Figure 2. Kaplan-Meier Plot of Progression-Free Survival – PALOMA-3 (Investigator Assessment, Intent-to-Treat Population) | ||
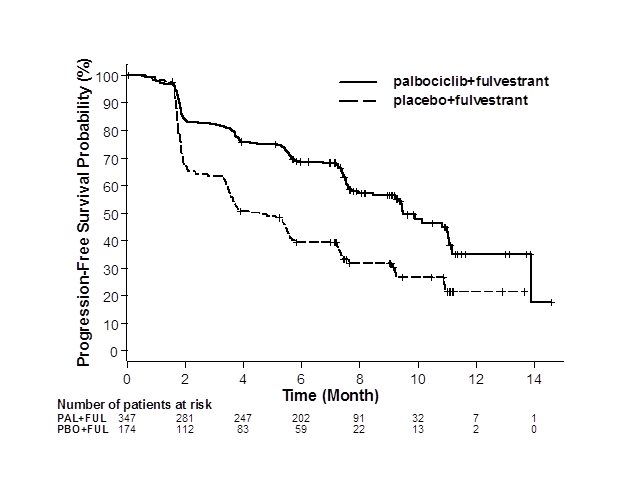 | ||
FUL=fulvestrant; PAL=palbociclib; PBO=placebo. | ||
Figure 3. Kaplan-Meier Plot of Overall Survival (Intent-to-Treat Population) – PALOMA-3 | ||
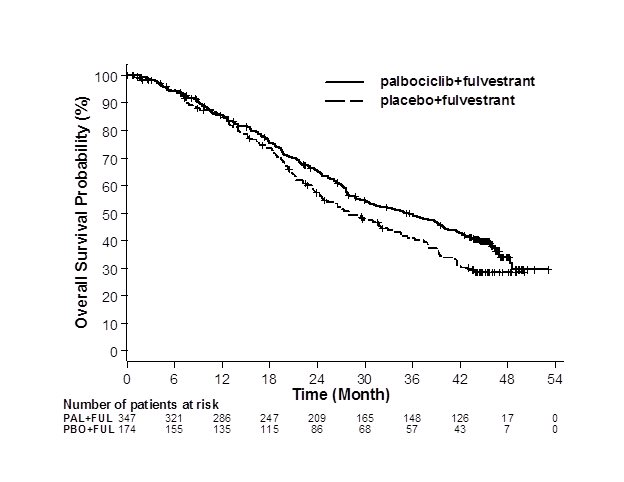 | ||
FUL=fulvestrant; PAL=palbociclib; PBO=placebo. | ||
Find IBRANCE® tablets medical information:
Find IBRANCE® tablets medical information:
IBRANCE® tablets Quick Finder
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
PALOMA-2: IBRANCE plus Letrozole
Patients with ER-positive, HER2-negative advanced or metastatic breast cancer for initial endocrine based therapy
PALOMA-2 was an international, randomized, double-blind, parallel-group, multicenter study of IBRANCE plus letrozole versus placebo plus letrozole conducted in postmenopausal women with ER-positive, HER2-negative advanced breast cancer who had not received previous systemic treatment for their advanced disease. A total of 666 patients were randomized 2:1 to IBRANCE plus letrozole or placebo plus letrozole. Randomization was stratified by disease site (visceral versus non-visceral), disease-free interval (de novo metastatic versus ≤12 months from the end of adjuvant treatment to disease recurrence versus >12 months from the end of adjuvant treatment to disease recurrence), and nature of prior (neo)adjuvant anticancer therapies (prior hormonal therapies versus no prior hormonal therapy). IBRANCE was given orally at a dose of 125 mg daily for 21 consecutive days followed by 7 days off treatment. Patients received study treatment until objective disease progression, symptomatic deterioration, unacceptable toxicity, death, or withdrawal of consent, whichever occurred first. The major efficacy outcome of the study was investigator-assessed progression-free survival (PFS) evaluated according to Response Evaluation Criteria in Solid Tumors Version 1.1 (RECIST). Additional efficacy outcome measures were confirmed overall response rate (ORR) as assessed by the investigator according to RECIST Version 1.1 and overall survival (OS).
Patients enrolled in this study had a median age of 62 years (range 28 to 89). The majority of patients were White (78%), and most patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1 (98%). Forty-eight percent of patients had received chemotherapy and 56% had received antihormonal therapy in the neoadjuvant or adjuvant setting prior to their diagnosis of advanced breast cancer. Thirty-seven percent of patients had no prior systemic therapy in the neoadjuvant or adjuvant setting. The majority of patients (97%) had metastatic disease. Twenty-three percent of patients had bone only disease, and 49% of patients had visceral disease.
Major efficacy results from PALOMA-2 are summarized in Table 8 and Figure 1. Consistent results were observed across patient subgroups of disease-free interval (DFI), disease site, and prior therapy. The treatment effect of the combination on PFS was also supported by an independent review of radiographs. Based on the prespecified final OS analysis conducted after 435 events, OS was not statistically significant.
| IBRANCE plus Letrozole | Placebo plus Letrozole | |
|---|---|---|
| CI=confidence interval; ITT=Intent-to-Treat; N=number of patients; NE=not estimable; OS=Overall survival; PFS=Progression‑free survival. | ||
Progression-free survival for ITT (investigator assessment) | N=444 | N=222 |
Number of PFS events (%) | 194 (43.7) | 137 (61.7) |
Median progression-free survival | 24.8 (22.1, NE) | 14.5 (12.9, 17.1) |
Hazard ratio (95% CI) and p-value | ||
Objective Response for patients with measurable disease (investigator assessment) | N=338 | N=171 |
Objective response rate‡ (%, 95% CI) | 55.3 (49.9, 60.7) | 44.4 (36.9, 52.2) |
Overall survival for ITT | N=444 | N=222 |
Number of OS events (%) | 287 (64.6) | 148 (66.7) |
Median OS (months, 95% CI) | 53.8 (49.8, 59.2) | 49.8 (42.3, 56.4) |
Hazard ratio (95% CI) and p‑value | ||
Figure 1. Kaplan-Meier Plot of Progression-Free Survival – PALOMA-2 (Investigator Assessment, Intent-to-Treat Population) | ||
 | ||
LET=letrozole; PAL=palbociclib; PBO=placebo. | ||
PALOMA-3: IBRANCE plus Fulvestrant
Patients with HR-positive, HER2-negative advanced or metastatic breast cancer who have had disease progression on or after prior adjuvant or metastatic endocrine therapy
PALOMA-3 was an international, randomized, double-blind, parallel group, multicenter study of IBRANCE plus fulvestrant versus placebo plus fulvestrant conducted in women with HR-positive, HER2-negative advanced breast cancer, regardless of their menopausal status, whose disease progressed on or after prior endocrine therapy. A total of 521 pre/postmenopausal women were randomized 2:1 to IBRANCE plus fulvestrant or placebo plus fulvestrant and stratified by documented sensitivity to prior hormonal therapy, menopausal status at study entry (pre/peri versus postmenopausal), and presence of visceral metastases. IBRANCE was given orally at a dose of 125 mg daily for 21 consecutive days followed by 7 days off treatment. Pre/perimenopausal women were enrolled in the study and received the LHRH agonist goserelin for at least 4 weeks prior to and for the duration of PALOMA-3. Patients continued to receive assigned treatment until objective disease progression, symptomatic deterioration, unacceptable toxicity, death, or withdrawal of consent, whichever occurred first. The major efficacy outcome of the study was investigator-assessed PFS evaluated according to RECIST 1.1.
Patients enrolled in this study had a median age of 57 years (range 29 to 88). The majority of patients on study were White (74%), all patients had an ECOG PS of 0 or 1, and 80% were postmenopausal. All patients had received prior systemic therapy, and 75% of patients had received a previous chemotherapy regimen. Twenty-five percent of patients had received no prior therapy in the metastatic disease setting, 60% had visceral metastases, and 23% had bone only disease.
The results from the investigator-assessed PFS and final OS from PALOMA-3 are summarized in Table 9. The relevant Kaplan-Meier plots are shown in Figures 2 and 3, respectively. Consistent PFS results were observed across patient subgroups of disease site, sensitivity to prior hormonal therapy, and menopausal status. After a median follow-up time of 45 months, the final OS results were not statistically significant.
| IBRANCE plus Fulvestrant | Placebo plus Fulvestrant | |
|---|---|---|
| CI=confidence interval; ITT=Intent-to-Treat; N=number of patients; OS=overall survival; PFS=progression-free survival. | ||
Progression-free survival for ITT | N=347 | N=174 |
Number of PFS events (%) | 145 (41.8) | 114 (65.5) |
Median PFS (months, 95% CI) | 9.5 (9.2, 11.0) | 4.6 (3.5, 5.6) |
Hazard ratio (95% CI) and p-value | 0.461 (0.360, 0.591), p<0.0001 | |
Objective Response for patients with measurable disease | N=267 | N=138 |
Objective response rate* (%, 95% CI) | 24.6 (19.6, 30.2) | 10.9 (6.2, 17.3) |
Overall survival for ITT | N=347 | N=174 |
Number of OS events (%) | 201 (57.9) | 109 (62.6) |
Median OS (months, 95% CI) | 34.9 (28.8, 40.0) | 28.0 (23.6, 34.6) |
Hazard ratio (95% CI) and p-value | ||
Figure 2. Kaplan-Meier Plot of Progression-Free Survival – PALOMA-3 (Investigator Assessment, Intent-to-Treat Population) | ||
 | ||
FUL=fulvestrant; PAL=palbociclib; PBO=placebo. | ||
Figure 3. Kaplan-Meier Plot of Overall Survival (Intent-to-Treat Population) – PALOMA-3 | ||
 | ||
FUL=fulvestrant; PAL=palbociclib; PBO=placebo. | ||
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.