heparin sodium in 5% dextrose injection 50 units/ml and 100 units/ml Description
11 DESCRIPTION
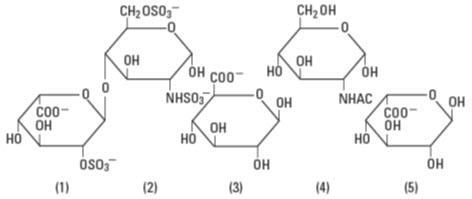
Heparin is a heterogeneous group of straight-chain anionic mucopolysaccharides, called glycosaminoglycans having anticoagulant properties. Although others may be present, the main sugars occurring in heparin are: (1) α-L-iduronic acid 2-sulfate, (2) 2-deoxy-2-sulfamino-α-D-glucose 6∙sulfate, (3) β-D-glucuronic acid, (4) 2-acetamido-2-deoxy-α-D-glucose, and (5) α-L-iduronic acid. These sugars are present in decreasing amounts, usually in the order (2)>(1)>(4)>(3)>(5), and are joined by glycosidic linkages, forming polymers of varying sizes. Heparin is strongly acidic because of its content of covalently linked sulfate and carboxylic acid groups. In heparin sodium, the acidic protons of the sulfate units are partially replaced by sodium ions.
Structure of Heparin Sodium (representative subunits):
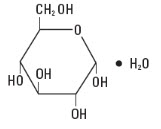
Dextrose, USP is chemically designated D-glucose, monohydrate C6H12O6 ∙ H2O, a hexose sugar freely soluble in water. It has the following structural formula:
Water for Injection, USP is chemically designated H2O.
Intravenous solutions with heparin sodium (derived from porcine intestinal mucosa) are sterile, nonpyrogenic fluids for intravenous administration. Each 100 mL contains heparin sodium 5,000 or 10,000 USP Units; dextrose, hydrous 5 g; citric acid, anhydrous, 51 mg and sodium citrate, dihydrate 334 mg added as buffers; sodium metabisulfite 20 mg added as an antioxidant. Each liter contains electrolytes sodium and citrate in amounts as listed in HOW SUPPLIED/STORAGE AND HANDLING Table. See Table for summary of contents and characteristics of this solution. The potency is determined by a biological assay using a USP reference standard based on units of heparin activity per milligram.
The flexible plastic container is fabricated either from a specially formulated nonplasticized, thermoplastic co-polyester (CR3) or from a polyolefin film. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions inside the plastic container also can leach out certain of its chemical components in very small amounts before the expiration period is attained. However, the safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers.
Find heparin sodium in 5% dextrose injection 50 units/ml and 100 units/ml medical information:
Find heparin sodium in 5% dextrose injection 50 units/ml and 100 units/ml medical information:
heparin sodium in 5% dextrose injection 50 units/ml and 100 units/ml Quick Finder
Health Professional Information
Description
11 DESCRIPTION
Heparin is a heterogeneous group of straight-chain anionic mucopolysaccharides, called glycosaminoglycans having anticoagulant properties. Although others may be present, the main sugars occurring in heparin are: (1) α-L-iduronic acid 2-sulfate, (2) 2-deoxy-2-sulfamino-α-D-glucose 6∙sulfate, (3) β-D-glucuronic acid, (4) 2-acetamido-2-deoxy-α-D-glucose, and (5) α-L-iduronic acid. These sugars are present in decreasing amounts, usually in the order (2)>(1)>(4)>(3)>(5), and are joined by glycosidic linkages, forming polymers of varying sizes. Heparin is strongly acidic because of its content of covalently linked sulfate and carboxylic acid groups. In heparin sodium, the acidic protons of the sulfate units are partially replaced by sodium ions.
Structure of Heparin Sodium (representative subunits):
Dextrose, USP is chemically designated D-glucose, monohydrate C6H12O6 ∙ H2O, a hexose sugar freely soluble in water. It has the following structural formula:
Water for Injection, USP is chemically designated H2O.
Intravenous solutions with heparin sodium (derived from porcine intestinal mucosa) are sterile, nonpyrogenic fluids for intravenous administration. Each 100 mL contains heparin sodium 5,000 or 10,000 USP Units; dextrose, hydrous 5 g; citric acid, anhydrous, 51 mg and sodium citrate, dihydrate 334 mg added as buffers; sodium metabisulfite 20 mg added as an antioxidant. Each liter contains electrolytes sodium and citrate in amounts as listed in HOW SUPPLIED/STORAGE AND HANDLING Table. See Table for summary of contents and characteristics of this solution. The potency is determined by a biological assay using a USP reference standard based on units of heparin activity per milligram.
The flexible plastic container is fabricated either from a specially formulated nonplasticized, thermoplastic co-polyester (CR3) or from a polyolefin film. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions inside the plastic container also can leach out certain of its chemical components in very small amounts before the expiration period is attained. However, the safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.