heparin sodium injection CARPUJECT Description
11 DESCRIPTION
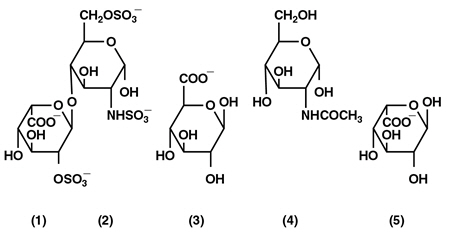
Heparin is a heterogenous group of straight-chain anionic mucopolysaccharides, called glycosaminoglycans, having anticoagulant properties. Although others may be present, the main sugars occurring in heparin are: (1) α-L-iduronic acid 2-sulfate, (2) 2-deoxy-2-sulfamino-α-D-glucose 6-sulfate, (3) β-D-glucuronic acid, (4) 2-acetamido-2-deoxy-α-D-glucose, and (5) α-L-iduronic acid. These sugars are present in decreasing amounts, usually in the order (2)> (1)> (4)> (3)> (5), and are joined by glycosidic linkages, forming polymers of varying sizes. Heparin is strongly acidic because of its content of covalently linked sulfate and carboxylic acid groups. In heparin sodium, the acidic protons of the sulfate units are partially replaced by sodium ions.
Structural formula of Heparin Sodium (representative subunits):
Heparin Sodium Injection, USP is a sterile solution of heparin sodium derived from porcine intestinal mucosa, standardized for anticoagulant activity. It is to be administered by intravenous or deep subcutaneous routes. The potency is determined by a biological assay using a USP reference standard based on units of heparin activity per milligram.
Carpuject™ sterile cartridge unit contain a sterile solution of Heparin Sodium Injection, USP.
Each mL contains 5,000 USP Units of heparin sodium and benzyl alcohol 1% as a preservative, in Water for Injection. The pH is adjusted between 5.0 to 7.5 with hydrochloric acid or sodium hydroxide.
Each 0.5 mL of Preservative-Free Heparin Sodium Injection contains 5,000 USP Units in Water for Injection. The pH is adjusted between 5.0 to 7.5 with hydrochloric acid or sodium hydroxide as required.
Find heparin sodium injection CARPUJECT medical information:
Find heparin sodium injection CARPUJECT medical information:
heparin sodium injection CARPUJECT Quick Finder
Health Professional Information
Description
11 DESCRIPTION
Heparin is a heterogenous group of straight-chain anionic mucopolysaccharides, called glycosaminoglycans, having anticoagulant properties. Although others may be present, the main sugars occurring in heparin are: (1) α-L-iduronic acid 2-sulfate, (2) 2-deoxy-2-sulfamino-α-D-glucose 6-sulfate, (3) β-D-glucuronic acid, (4) 2-acetamido-2-deoxy-α-D-glucose, and (5) α-L-iduronic acid. These sugars are present in decreasing amounts, usually in the order (2)> (1)> (4)> (3)> (5), and are joined by glycosidic linkages, forming polymers of varying sizes. Heparin is strongly acidic because of its content of covalently linked sulfate and carboxylic acid groups. In heparin sodium, the acidic protons of the sulfate units are partially replaced by sodium ions.
Structural formula of Heparin Sodium (representative subunits):

Heparin Sodium Injection, USP is a sterile solution of heparin sodium derived from porcine intestinal mucosa, standardized for anticoagulant activity. It is to be administered by intravenous or deep subcutaneous routes. The potency is determined by a biological assay using a USP reference standard based on units of heparin activity per milligram.
Carpuject™ sterile cartridge unit contain a sterile solution of Heparin Sodium Injection, USP.
Each mL contains 5,000 USP Units of heparin sodium and benzyl alcohol 1% as a preservative, in Water for Injection. The pH is adjusted between 5.0 to 7.5 with hydrochloric acid or sodium hydroxide.
Each 0.5 mL of Preservative-Free Heparin Sodium Injection contains 5,000 USP Units in Water for Injection. The pH is adjusted between 5.0 to 7.5 with hydrochloric acid or sodium hydroxide as required.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.