gemcitabine injection powder 2GM Description
11 DESCRIPTION
Gemcitabine is a nucleoside metabolic inhibitor. Gemcitabine hydrochloride is 2´-deoxy-2´,2´-difluorocytidine monohydrochloride (β-isomer) with the following structural formula:
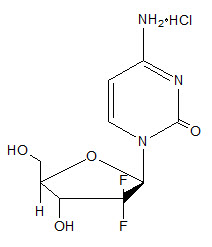
The empirical formula for gemcitabine hydrochloride is C9H11F2N3O4 ∙ HCl. It has a molecular weight of 299.66 g/mol.
Gemcitabine hydrochloride is soluble in water, slightly soluble in methanol, and practically insoluble in ethanol and polar organic solvents.
Gemcitabine hydrochloride is a sterile white to off-white lyophilized powder and available as 2 g single-dose vials for intravenous use only. Each 2 g vial contains 2 g of gemcitabine hydrochloride (expressed as free base), 2 g mannitol, and 125 mg sodium acetate. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment.
Find gemcitabine injection powder 2GM medical information:
Find gemcitabine injection powder 2GM medical information:
gemcitabine injection powder 2GM Quick Finder
Health Professional Information
Description
11 DESCRIPTION
Gemcitabine is a nucleoside metabolic inhibitor. Gemcitabine hydrochloride is 2´-deoxy-2´,2´-difluorocytidine monohydrochloride (β-isomer) with the following structural formula:

The empirical formula for gemcitabine hydrochloride is C9H11F2N3O4 ∙ HCl. It has a molecular weight of 299.66 g/mol.
Gemcitabine hydrochloride is soluble in water, slightly soluble in methanol, and practically insoluble in ethanol and polar organic solvents.
Gemcitabine hydrochloride is a sterile white to off-white lyophilized powder and available as 2 g single-dose vials for intravenous use only. Each 2 g vial contains 2 g of gemcitabine hydrochloride (expressed as free base), 2 g mannitol, and 125 mg sodium acetate. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.