11 DESCRIPTION
Dacomitinib is an oral kinase inhibitor with a molecular formula of C24H25ClFN5O2 ∙ H2O and a molecular weight of 487.95 Daltons. The chemical name is: (2E)-N-{4-[(3-Chloro-4-fluorophenyl)amino]-7-methoxyquinazolin-6-yl}-4-(piperidin-1-yl)but-2-enamide monohydrate and its structural formula is:
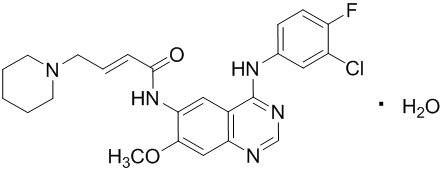
Dacomitinib is a white to pale yellow powder.
VIZIMPRO tablets contain 45, 30, or 15 mg of dacomitinib with the following inactive ingredients in the tablet core; lactose monohydrate, microcrystalline cellulose, sodium starch glycolate, and magnesium stearate. The film coating consists of Opadry II Blue 85F30716 containing: Polyvinyl alcohol – partially hydrolyzed, Talc, Titanium dioxide, Macrogol/PEG 3350, and FD&C Blue #2/Indigo Carmine Aluminum Lake.