VFEND® Instructions For Use
(voriconazole)
INSTRUCTIONS FOR USE
VFEND (VEE-fend)
(voriconazole)
for oral suspension
Read this Instructions for Use before you start taking VFEND and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
Important information:
- Follow your healthcare provider's instructions for the dose of VFEND to take.
- Ask your healthcare provider or pharmacist if you are not sure how to take VFEND.
- VFEND for oral suspension is a liquid form of VFEND. Your pharmacist will mix (reconstitute) the medicine before it is dispensed to you. If VFEND is still in powder form, do not use it. Return it to your pharmacist.
- Always use the oral dispenser provided with VFEND to make sure you measure the right amount of VFEND.
- Shake the closed bottle of mixed (reconstituted) oral suspension well for about 10 seconds before each use.
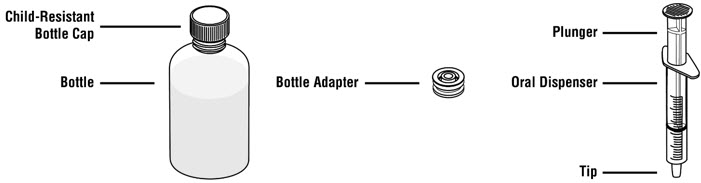
Each pack contains:
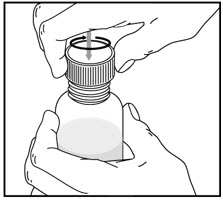
How to prepare the bottle and take VFEND:
| 1. |
| 2. |
| 3. |
|
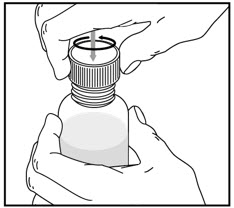
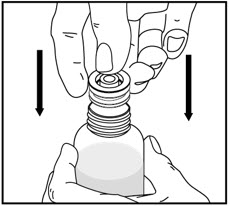
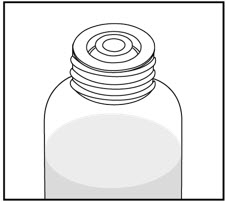
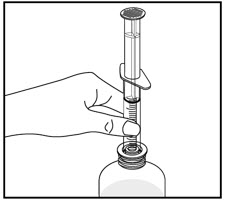
| Remove the child-resistant bottle cap by pushing down while twisting the cap to the left (counter-clockwise). | Push the bottle adapter firmly into the bottle (if your pharmacist has not already inserted the bottle adapter). If the bottle adapter is missing, contact your pharmacist. | Important: Bottle adapter must be fully inserted before use. | |||
| Do not remove the bottle adapter after it is inserted. | |||||
| 4. |
| 5. |
| 6. |
|
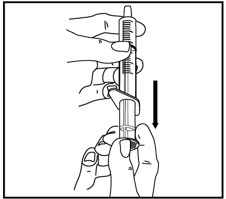
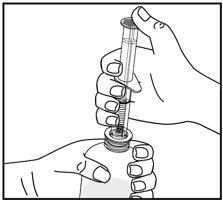
| Pull back on the oral dispenser plunger to your prescribed dose. | Insert the tip of the oral dispenser into the bottle adapter. | While holding the bottle with 1 hand, push down on the oral dispenser plunger with your other hand to push air into the bottle. | |||
| 7. |
| 8. |
| 9. |
|
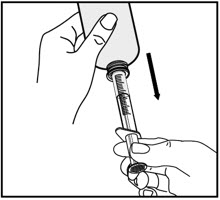
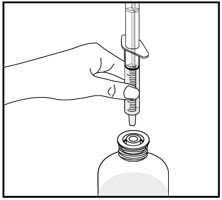
| Turn the bottle upside down and slowly pull back on the oral dispenser plunger to withdraw your prescribed dose of medicine. | Turn the bottle back upright with the oral dispenser still in place. Remove the tip of the oral dispenser from the bottle adapter. | Screw the bottle cap back on the bottle tightly by turning the cap to the right (clockwise). | |||
| Place the tip of the oral dispenser in your mouth and point the tip of the oral dispenser towards the inside of the cheek. Slowly push the plunger until all the medicine is given. Do not squirt the medicine out quickly. This may cause you to choke. | Do not remove the bottle adapter. The bottle cap will fit over it. | ||||
| If the medicine is to be given to a child, keep your child in an upright position while giving the medicine. |
Rinse the oral dispenser after each use.
- Pull the plunger out of the oral dispenser and wash both parts with warm soapy water.
- Rinse both parts with water and allow to air dry after each use.
- After air drying, push the plunger back into the oral dispenser.
- Store the oral dispenser with VFEND oral suspension in a clean safe place.
How should I store VFEND oral suspension?
- Store VFEND oral suspension at room temperature between 59°F to 86°F (15°C to 30°C).
- Do not refrigerate or freeze.
- Keep the bottle cap tightly closed.
- Use VFEND oral suspension within 14 days after it has been mixed (reconstituted) by the pharmacist. The pharmacist will write the expiration date on the bottle label (the expiration date of the oral suspension is 14 days from the date it was mixed (reconstituted) by the pharmacist). Throw away (discard) any unused VFEND after the expiration date.
- Keep VFEND and all medicines out of the reach of children.

This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
LAB-1348-5.0
Revised: 8/2022
Find VFEND® medical information:
Find VFEND® medical information:
VFEND® Quick Finder
Health Professional Information
Instructions For Use
INSTRUCTIONS FOR USE
VFEND (VEE-fend)
(voriconazole)
for oral suspension
Read this Instructions for Use before you start taking VFEND and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
Important information:
- Follow your healthcare provider's instructions for the dose of VFEND to take.
- Ask your healthcare provider or pharmacist if you are not sure how to take VFEND.
- VFEND for oral suspension is a liquid form of VFEND. Your pharmacist will mix (reconstitute) the medicine before it is dispensed to you. If VFEND is still in powder form, do not use it. Return it to your pharmacist.
- Always use the oral dispenser provided with VFEND to make sure you measure the right amount of VFEND.
- Shake the closed bottle of mixed (reconstituted) oral suspension well for about 10 seconds before each use.
Each pack contains:

How to prepare the bottle and take VFEND:
| 1. |
| 2. |
| 3. |
|
| Remove the child-resistant bottle cap by pushing down while twisting the cap to the left (counter-clockwise). | Push the bottle adapter firmly into the bottle (if your pharmacist has not already inserted the bottle adapter). If the bottle adapter is missing, contact your pharmacist. | Important: Bottle adapter must be fully inserted before use. | |||
| Do not remove the bottle adapter after it is inserted. | |||||
| 4. |
| 5. |
| 6. |
|
| Pull back on the oral dispenser plunger to your prescribed dose. | Insert the tip of the oral dispenser into the bottle adapter. | While holding the bottle with 1 hand, push down on the oral dispenser plunger with your other hand to push air into the bottle. | |||
| 7. |
| 8. |
| 9. |
|
| Turn the bottle upside down and slowly pull back on the oral dispenser plunger to withdraw your prescribed dose of medicine. | Turn the bottle back upright with the oral dispenser still in place. Remove the tip of the oral dispenser from the bottle adapter. | Screw the bottle cap back on the bottle tightly by turning the cap to the right (clockwise). | |||
| Place the tip of the oral dispenser in your mouth and point the tip of the oral dispenser towards the inside of the cheek. Slowly push the plunger until all the medicine is given. Do not squirt the medicine out quickly. This may cause you to choke. | Do not remove the bottle adapter. The bottle cap will fit over it. | ||||
| If the medicine is to be given to a child, keep your child in an upright position while giving the medicine. |
Rinse the oral dispenser after each use.
- Pull the plunger out of the oral dispenser and wash both parts with warm soapy water.
- Rinse both parts with water and allow to air dry after each use.
- After air drying, push the plunger back into the oral dispenser.
- Store the oral dispenser with VFEND oral suspension in a clean safe place.
How should I store VFEND oral suspension?
- Store VFEND oral suspension at room temperature between 59°F to 86°F (15°C to 30°C).
- Do not refrigerate or freeze.
- Keep the bottle cap tightly closed.
- Use VFEND oral suspension within 14 days after it has been mixed (reconstituted) by the pharmacist. The pharmacist will write the expiration date on the bottle label (the expiration date of the oral suspension is 14 days from the date it was mixed (reconstituted) by the pharmacist). Throw away (discard) any unused VFEND after the expiration date.
- Keep VFEND and all medicines out of the reach of children.

This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
LAB-1348-5.0
Revised: 8/2022
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.