DESCRIPTION
Verapamil hydrochloride is a calcium antagonist or slow-channel inhibitor. Verapamil Hydrochloride Injection, USP is a sterile, nonpyrogenic solution containing verapamil hydrochloride 2.5 mg/mL and sodium chloride 8.5 mg/mL in water for injection. The solution contains no bacteriostat or antimicrobial agent and is intended for single-dose intravenous administration. May contain hydrochloric acid for pH adjustment; pH is 4.0 to 6.5.
The chemical name of Verapamil Hydrochloride, USP is benzeneacetonitrile, α-[3-[{2-(3,4-dimethoxyphenyl)ethyl} methylamino] propyl]-3,4-dimethoxy-α-(1-methylethyl) hydrochloride. Verapamil hydrochloride is a white or practically white crystalline powder. It is practically odorless and has a bitter taste. It is soluble in water; freely soluble in chloroform; sparingly soluble in alcohol; practically insoluble in ether. It has the following structural formula:
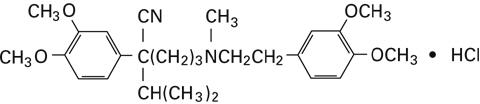
Molecular weight: 491.07
Molecular formula: C27H38N2O4 • HCl
Verapamil hydrochloride is not chemically related to other antiarrhythmic drugs.
The plastic syringe is molded from a specially formulated polypropylene. Water permeates from inside the container at an extremely slow rate which will have an insignificant effect on solution concentration over the expected shelf life. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the syringe material.