TIKOSYN® Dosage and Administration
(dofetilide)
DOSAGE AND ADMINISTRATION
- •
- Therapy with TIKOSYN must be initiated (and, if necessary, re-initiated) in a setting that provides continuous electrocardiographic (ECG) monitoring and in the presence of personnel trained in the management of serious ventricular arrhythmias. Patients should continue to be monitored in this way for a minimum of three days. Additionally, patients should not be discharged within 12 hours of electrical or pharmacological conversion to normal sinus rhythm.
- •
- The dose of TIKOSYN must be individualized according to calculated creatinine clearance and QTc. (QT interval should be used if the heart rate is <60 beats per minute. There are no data on use of TIKOSYN when the heart rate is <50 beats per minute.) The usual recommended dose of TIKOSYN is 500 mcg BID, as modified by the dosing algorithm described below. For consideration of a lower dose, see Special Considerations below.
- •
- Serum potassium should be maintained within the normal range before TIKOSYN treatment is initiated and should be maintained within the normal range while the patient remains on TIKOSYN therapy. (See WARNINGS, Hypokalemia and Potassium-Depleting Diuretics). In clinical trials, potassium levels were generally maintained above 3.6–4.0 mEq/L.
- •
- Patients with atrial fibrillation should be anticoagulated according to usual medical practice prior to electrical or pharmacological cardioversion. Anticoagulant therapy may be continued after cardioversion according to usual medical practice for the treatment of people with AF. Hypokalemia should be corrected before initiation of TIKOSYN therapy (see WARNINGS, Ventricular Arrhythmia).
- •
- Patients to be discharged on TIKOSYN therapy from an inpatient setting as described above must have an adequate supply of TIKOSYN, at the patient's individualized dose, to allow uninterrupted dosing until the patient can fill a TIKOSYN prescription.
Instructions for Individualized Dose Initiation
Initiation of TIKOSYN Therapy
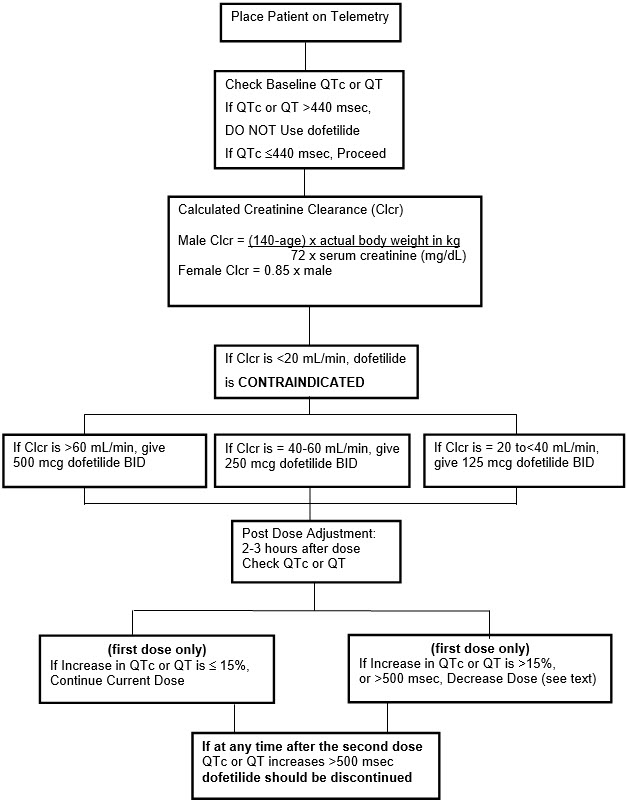
Step 1. Electrocardiographic assessment: Prior to administration of the first dose, the QTc or QT must be checked using an average of 5–10 beats. If the QTc or QT is greater than 440 msec (500 msec in patients with ventricular conduction abnormalities), TIKOSYN is contraindicated. If heart rate is less than 60 beats per minute, QT interval should be used. Proceed to Step 2 if the QTc or QT is 440 msec. Patients with heart rates <50 beats per minute have not been studied.
Step 2. Calculation of creatinine clearance: Prior to the administration of the first dose, the patient's creatinine clearance must be calculated using the following formula:
creatinine clearance (male) = | (140-age) × actual body weight in kg |
creatinine clearance (female) = | (140-age) × actual body weight in kg × 0.85 |
When serum creatinine is given in µmol/L, divide the value by 88.4 (1 mg/dL = 88.4 µmol/L).
Step 3. Starting Dose: The starting dose of TIKOSYN is determined as follows:
| Calculated Creatinine Clearance | TIKOSYN Dose |
|---|---|
>60 mL/min | 500 mcg twice daily |
40 to 60 mL/min | 250 mcg twice daily |
20 to <40 mL/min | 125 mcg twice daily |
<20 mL/min | Tikosyn is contraindicated |
Step 4. Administer the adjusted TIKOSYN dose and begin continuous ECG monitoring.
Step 5. At 2–3 hours after administering the first dose of Tikosyn, determine the QTc or QT (if heart rate is less than 60 beats per minute). If the QTc or QT has increased by greater than 15% compared to the baseline established in Step 1 OR if the QTc or QT is greater than 500 msec (550 msec in patients with ventricular conduction abnormalities), subsequent dosing should be adjusted as follows:
| If the Starting Dose Based on Creatinine Clearance is: | Then the Adjusted Dose (for QTc or QT Prolongation) is: | |
|---|---|---|
500 mcg twice daily | 250 mcg twice daily | |
250 mcg twice daily | 125 mcg twice daily | |
125 mcg twice daily | 125 mcg once a day |
Step 6. At 2–3 hours after each subsequent dose of Tikosyn, determine the QTc or QT (if heart rate is less than 60 beats per minute) (for in-hospital doses 2–5). No further down titration of Tikosyn based on QTc or QT is recommended.
NOTE: If at any time after the second dose of Tikosyn is given the QTc or QT is greater than 500 msec (550 msec in patients with ventricular conduction abnormalities), Tikosyn should be discontinued.
Step 7. Patients are to be continuously monitored by ECG for a minimum of three days, or for a minimum of 12 hours after electrical or pharmacological conversion to normal sinus rhythm, whichever is greater.
The steps described above are summarized in the following diagram:
Maintenance of TIKOSYN Therapy
Renal function and QTc or QT (if heart rate is less than 60 beats per minute) should be re-evaluated every three months or as medically warranted. If QTc or QT exceeds 500 milliseconds (550 msec in patients with ventricular conduction abnormalities), TIKOSYN therapy should be discontinued and patients should be carefully monitored until QTc or QT returns to baseline levels. If renal function deteriorates, adjust dose as described in Initiation of TIKOSYN Therapy, Step 3.
Special Considerations
Consideration of a Dose Lower than that Determined by the Algorithm:
The dosing algorithm shown above should be used to determine the individualized dose of TIKOSYN. In clinical trials (see CLINICAL STUDIES), the highest dose of 500 mcg BID of TIKOSYN as modified by the dosing algorithm led to greater effectiveness than lower doses of 125 or 250 mcg BID as modified by the dosing algorithm. The risk of Torsade de Pointes, however, is related to dose as well as to patient characteristics (see WARNINGS). Physicians, in consultation with their patients, may therefore in some cases choose doses lower than determined by the algorithm. It is critically important that if at any time this lower dose is increased, the patient needs to be rehospitalized for three days. Previous toleration of higher doses does not eliminate the need for rehospitalization.
The maximum recommended dose in patients with a calculated creatinine clearance greater than 60 mL/min is 500 mcg BID; doses greater than 500 mcg BID have been associated with an increased incidence of Torsade de Pointes.
A patient who misses a dose should NOT double the next dose. The next dose should be taken at the usual time.
Cardioversion:
If patients do not convert to normal sinus rhythm within 24 hours of initiation of TIKOSYN therapy, electrical conversion should be considered. Patients continuing on TIKOSYN after successful electrical cardioversion should continue to be monitored by electrocardiography for 12 hours post cardioversion, or a minimum of 3 days after initiation of TIKOSYN therapy, whichever is greater.
Switch to TIKOSYN from Class I or other Class III Antiarrhythmic Therapy
Before initiating TIKOSYN therapy, previous antiarrhythmic therapy should be withdrawn under careful monitoring for a minimum of three (3) plasma half-lives. Because of the unpredictable pharmacokinetics of amiodarone, TIKOSYN should not be initiated following amiodarone therapy until amiodarone plasma levels are below 0.3 mcg/mL or until amiodarone has been withdrawn for at least three months.
Find TIKOSYN® medical information:
Find TIKOSYN® medical information:
TIKOSYN® Quick Finder
Health Professional Information
Dosage and Administration
DOSAGE AND ADMINISTRATION
- •
- Therapy with TIKOSYN must be initiated (and, if necessary, re-initiated) in a setting that provides continuous electrocardiographic (ECG) monitoring and in the presence of personnel trained in the management of serious ventricular arrhythmias. Patients should continue to be monitored in this way for a minimum of three days. Additionally, patients should not be discharged within 12 hours of electrical or pharmacological conversion to normal sinus rhythm.
- •
- The dose of TIKOSYN must be individualized according to calculated creatinine clearance and QTc. (QT interval should be used if the heart rate is <60 beats per minute. There are no data on use of TIKOSYN when the heart rate is <50 beats per minute.) The usual recommended dose of TIKOSYN is 500 mcg BID, as modified by the dosing algorithm described below. For consideration of a lower dose, see Special Considerations below.
- •
- Serum potassium should be maintained within the normal range before TIKOSYN treatment is initiated and should be maintained within the normal range while the patient remains on TIKOSYN therapy. (See WARNINGS, Hypokalemia and Potassium-Depleting Diuretics). In clinical trials, potassium levels were generally maintained above 3.6–4.0 mEq/L.
- •
- Patients with atrial fibrillation should be anticoagulated according to usual medical practice prior to electrical or pharmacological cardioversion. Anticoagulant therapy may be continued after cardioversion according to usual medical practice for the treatment of people with AF. Hypokalemia should be corrected before initiation of TIKOSYN therapy (see WARNINGS, Ventricular Arrhythmia).
- •
- Patients to be discharged on TIKOSYN therapy from an inpatient setting as described above must have an adequate supply of TIKOSYN, at the patient's individualized dose, to allow uninterrupted dosing until the patient can fill a TIKOSYN prescription.
Instructions for Individualized Dose Initiation
Initiation of TIKOSYN Therapy
Step 1. Electrocardiographic assessment: Prior to administration of the first dose, the QTc or QT must be checked using an average of 5–10 beats. If the QTc or QT is greater than 440 msec (500 msec in patients with ventricular conduction abnormalities), TIKOSYN is contraindicated. If heart rate is less than 60 beats per minute, QT interval should be used. Proceed to Step 2 if the QTc or QT is 440 msec. Patients with heart rates <50 beats per minute have not been studied.
Step 2. Calculation of creatinine clearance: Prior to the administration of the first dose, the patient's creatinine clearance must be calculated using the following formula:
creatinine clearance (male) = | (140-age) × actual body weight in kg |
creatinine clearance (female) = | (140-age) × actual body weight in kg × 0.85 |
When serum creatinine is given in µmol/L, divide the value by 88.4 (1 mg/dL = 88.4 µmol/L).
Step 3. Starting Dose: The starting dose of TIKOSYN is determined as follows:
| Calculated Creatinine Clearance | TIKOSYN Dose |
|---|---|
>60 mL/min | 500 mcg twice daily |
40 to 60 mL/min | 250 mcg twice daily |
20 to <40 mL/min | 125 mcg twice daily |
<20 mL/min | Tikosyn is contraindicated |
Step 4. Administer the adjusted TIKOSYN dose and begin continuous ECG monitoring.
Step 5. At 2–3 hours after administering the first dose of Tikosyn, determine the QTc or QT (if heart rate is less than 60 beats per minute). If the QTc or QT has increased by greater than 15% compared to the baseline established in Step 1 OR if the QTc or QT is greater than 500 msec (550 msec in patients with ventricular conduction abnormalities), subsequent dosing should be adjusted as follows:
| If the Starting Dose Based on Creatinine Clearance is: | Then the Adjusted Dose (for QTc or QT Prolongation) is: | |
|---|---|---|
500 mcg twice daily | 250 mcg twice daily | |
250 mcg twice daily | 125 mcg twice daily | |
125 mcg twice daily | 125 mcg once a day |
Step 6. At 2–3 hours after each subsequent dose of Tikosyn, determine the QTc or QT (if heart rate is less than 60 beats per minute) (for in-hospital doses 2–5). No further down titration of Tikosyn based on QTc or QT is recommended.
NOTE: If at any time after the second dose of Tikosyn is given the QTc or QT is greater than 500 msec (550 msec in patients with ventricular conduction abnormalities), Tikosyn should be discontinued.
Step 7. Patients are to be continuously monitored by ECG for a minimum of three days, or for a minimum of 12 hours after electrical or pharmacological conversion to normal sinus rhythm, whichever is greater.
The steps described above are summarized in the following diagram:
Maintenance of TIKOSYN Therapy
Renal function and QTc or QT (if heart rate is less than 60 beats per minute) should be re-evaluated every three months or as medically warranted. If QTc or QT exceeds 500 milliseconds (550 msec in patients with ventricular conduction abnormalities), TIKOSYN therapy should be discontinued and patients should be carefully monitored until QTc or QT returns to baseline levels. If renal function deteriorates, adjust dose as described in Initiation of TIKOSYN Therapy, Step 3.
Special Considerations
Consideration of a Dose Lower than that Determined by the Algorithm:
The dosing algorithm shown above should be used to determine the individualized dose of TIKOSYN. In clinical trials (see CLINICAL STUDIES), the highest dose of 500 mcg BID of TIKOSYN as modified by the dosing algorithm led to greater effectiveness than lower doses of 125 or 250 mcg BID as modified by the dosing algorithm. The risk of Torsade de Pointes, however, is related to dose as well as to patient characteristics (see WARNINGS). Physicians, in consultation with their patients, may therefore in some cases choose doses lower than determined by the algorithm. It is critically important that if at any time this lower dose is increased, the patient needs to be rehospitalized for three days. Previous toleration of higher doses does not eliminate the need for rehospitalization.
The maximum recommended dose in patients with a calculated creatinine clearance greater than 60 mL/min is 500 mcg BID; doses greater than 500 mcg BID have been associated with an increased incidence of Torsade de Pointes.
A patient who misses a dose should NOT double the next dose. The next dose should be taken at the usual time.
Cardioversion:
If patients do not convert to normal sinus rhythm within 24 hours of initiation of TIKOSYN therapy, electrical conversion should be considered. Patients continuing on TIKOSYN after successful electrical cardioversion should continue to be monitored by electrocardiography for 12 hours post cardioversion, or a minimum of 3 days after initiation of TIKOSYN therapy, whichever is greater.
Switch to TIKOSYN from Class I or other Class III Antiarrhythmic Therapy
Before initiating TIKOSYN therapy, previous antiarrhythmic therapy should be withdrawn under careful monitoring for a minimum of three (3) plasma half-lives. Because of the unpredictable pharmacokinetics of amiodarone, TIKOSYN should not be initiated following amiodarone therapy until amiodarone plasma levels are below 0.3 mcg/mL or until amiodarone has been withdrawn for at least three months.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.