DESCRIPTION
Tham Solution (tromethamine injection) is a sterile, non-pyrogenic 0.3 M solution of tromethamine, adjusted to a pH of approximately 8.6 with glacial acetic acid. It is administered by intravenous injection, by addition to ACD blood for priming cardiac bypass equipment and by injection into the ventricular cavity during cardiac arrest.
Each 100 mL contains tromethamine 3.6 g (30 mEq) in water for injection. The solution is hypertonic 389 mOsmol/L (calc.). pH 8.6 (8.4-8.7).
The solution contains no bacteriostat, antimicrobial agent or added buffer (except acetic acid for pH adjustment) and is intended only for use as a single-dose injection. When smaller doses are required the unused portion should be discarded.
Tham solution is a parenteral systemic alkalizer and fluid replenisher.
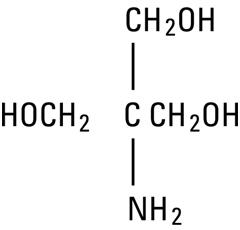
Tromethamine, USP (sometimes called “tris” or “tris buffer”) is chemically designated 2-amino-2-(hydroxymethyl)-1, 3-propanediol, a solid readily soluble in water, also classified as an organic amine buffer. It has the following structural formula:
Water for Injection, USP is chemically designated H20.