11 DESCRIPTION
Pegvisomant is an analog of human growth hormone (GH) of recombinant DNA origin that acts as a GH receptor antagonist.
It contains 191 amino acid residues. The molecular weight of pegvisomant is 22 kDa. The molecular weight of the PEG portion of pegvisomant is approximately 5 kDa. The predominant molecular weights of pegvisomant are thus approximately 42, 47, and 52 kDa. The schematic shows the amino acid sequence of the pegvisomant protein (PEG polymers are shown attached to the 5 most probable attachment sites). Pegvisomant is synthesized by a specific strain of Escherichia coli bacteria that has been genetically modified by the addition of a plasmid that carries a gene for GH receptor antagonist.
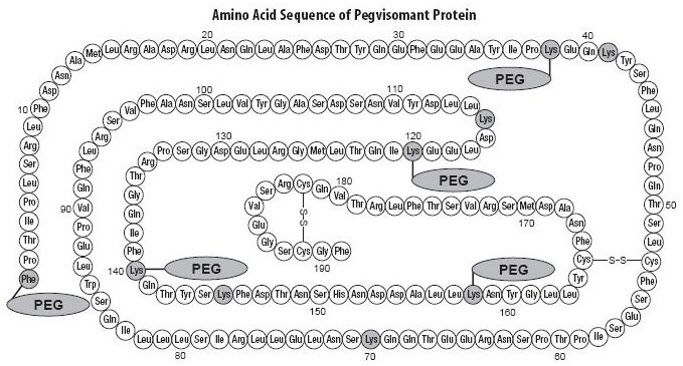 |
Stippled residues indicate PEG attachment sites (Phe1, Lys38, Lys41, Lys70, Lys115, Lys120, Lys140, Lys145, Lys158) |
Shown below are the amino acid substitutions in pegvisomant, relative to human GH.
| hGH | Pegvisomant |
|---|
His18 | Asp18 |
Ala21 | Asn21 |
Gly120 | Lys120 |
Arg167 | Asn167 |
Lys168 | Ala168 |
Asp171 | Ser171 |
Lys172 | Arg172 |
Glu174 | Ser174 |
Ile179 | Thr179 |
SOMAVERT (pegvisomant) for injection is a sterile, white lyophilized powder intended for subcutaneous injection after reconstitution. SOMAVERT is supplied in packages that include a single-dose prefilled syringe containing 1 mL of Sterile Water for Injection, USP, that is a sterile, nonpyrogenic preparation of water for injection that contains no bacteriostat, antimicrobial agent, or added buffer, to be used as a diluent.
SOMAVERT is available in single-dose sterile vials containing 10 mg, 15 mg, 20 mg, 25 mg or 30 mg of pegvisomant. SOMAVERT 10 mg, 15 mg, and 20 mg vials also contain glycine (1.36 mg), mannitol (36 mg), sodium dihydrogen phosphate monohydrate (0.36 mg), and sodium phosphate dibasic anhydrous (1.04 mg). After reconstitution with 1 mL of Water for Injection, USP, the resulting concentration is 10 mg/mL, 15 mg/mL and 20 mg/mL, respectively, with a pH of 7.1 – 7.7.
SOMAVERT 25 mg vial also contains glycine (1.7 mg), mannitol (45 mg), sodium dihydrogen phosphate monohydrate (0.45 mg), and sodium phosphate dibasic anhydrous (1.3 mg). After reconstitution with 1 mL of Water for Injection, USP, the resulting concentration is 25 mg/mL with a pH of 7.1 – 7.7.
SOMAVERT 30 mg vial also contains glycine (2.04 mg), mannitol (54 mg), sodium dihydrogen phosphate monohydrate (0.54 mg), and sodium phosphate dibasic anhydrous (1.56 mg). After reconstitution with 1 mL of Water for Injection, USP, the resulting concentration is 30 mg/mL with a pH of 7.1 – 7.7.