SOLU-MEDROL® Description
(methylprednisolone sodium succinate)
DESCRIPTION
SOLU-MEDROL Sterile Powder is an anti-inflammatory glucocorticoid, which contains methylprednisolone sodium succinate as the active ingredient. Methylprednisolone sodium succinate, USP, is the sodium succinate ester of methylprednisolone, and it occurs as a white, or nearly white, odorless hygroscopic, amorphous solid. It is very soluble in water and in alcohol; it is insoluble in chloroform and is very slightly soluble in acetone.
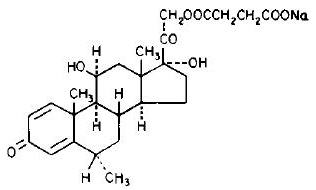
The chemical name for methylprednisolone sodium succinate is pregna-1,4-diene-3,20-dione,21-(3-carboxy-1-oxopropoxy)-11,17-dihydroxy-6-methyl-monosodium salt, (6α, 11β), and the molecular weight is 496.53. The structural formula is represented below:
Methylprednisolone sodium succinate is soluble in water; it may be administered in a small volume of diluent and is well suited for intravenous use in situations where high blood levels of methylprednisolone are required rapidly.
SOLU-MEDROL is available in preservative and preservative-free formulations:
40 mg Act-O-Vial System (Single-Dose Vial)—Each mL (when mixed) contains methylprednisolone sodium succinate equivalent to 40 mg methylprednisolone; also |
125 mg Act-O-Vial System (Single-Dose Vial)—Each 2 mL (when mixed) contains methylprednisolone sodium succinate equivalent to 125 mg methylprednisolone; also |
500 mg Act-O-Vial System (Single-Dose Vial)—Each 4 mL (when mixed) contains methylprednisolone sodium succinate equivalent to 500 mg methylprednisolone; also |
1 gram Act-O-Vial System (Single-Dose Vial)—Each 8 mL (when mixed) contains methylprednisolone sodium succinate equivalent to 1 gram methylprednisolone; also |
500 mg Vial—Each 8 mL (when mixed as directed) contains methylprednisolone sodium succinate equivalent to 500 mg methylprednisolone; also 6.4 mg monobasic sodium phosphate anhydrous; 69.6 mg dibasic sodium phosphate dried. |
1 gram Vial—Each 16 mL (when mixed as directed) contains methylprednisolone sodium succinate equivalent to 1 gram methylprednisolone; also 12.8 mg monobasic sodium phosphate anhydrous; 139.2 mg dibasic sodium phosphate dried. |
2 gram Vial—Each 30.6 mL (when mixed as directed) contains methylprednisolone sodium succinate equivalent to 2 grams methylprednisolone; also 25.6 mg monobasic sodium phosphate anhydrous; 278 mg dibasic sodium phosphate dried. |
2 gram Vial with Diluent—Each 30.6 mL (when mixed as directed) contains methylprednisolone sodium succinate equivalent to 2 grams methylprednisolone; also 25.6 mg monobasic sodium phosphate anhydrous; 278 mg dibasic sodium phosphate dried. |
IMPORTANT — Use only the accompanying diluent |
When necessary, the pH of each formula was adjusted with sodium hydroxide so that the pH of the reconstituted solution is within the USP specified range of 7 to 8.
Find SOLU-MEDROL® medical information:
Find SOLU-MEDROL® medical information:
SOLU-MEDROL® Quick Finder
Health Professional Information
Description
DESCRIPTION
SOLU-MEDROL Sterile Powder is an anti-inflammatory glucocorticoid, which contains methylprednisolone sodium succinate as the active ingredient. Methylprednisolone sodium succinate, USP, is the sodium succinate ester of methylprednisolone, and it occurs as a white, or nearly white, odorless hygroscopic, amorphous solid. It is very soluble in water and in alcohol; it is insoluble in chloroform and is very slightly soluble in acetone.
The chemical name for methylprednisolone sodium succinate is pregna-1,4-diene-3,20-dione,21-(3-carboxy-1-oxopropoxy)-11,17-dihydroxy-6-methyl-monosodium salt, (6α, 11β), and the molecular weight is 496.53. The structural formula is represented below:
Methylprednisolone sodium succinate is soluble in water; it may be administered in a small volume of diluent and is well suited for intravenous use in situations where high blood levels of methylprednisolone are required rapidly.
SOLU-MEDROL is available in preservative and preservative-free formulations:
40 mg Act-O-Vial System (Single-Dose Vial)—Each mL (when mixed) contains methylprednisolone sodium succinate equivalent to 40 mg methylprednisolone; also |
125 mg Act-O-Vial System (Single-Dose Vial)—Each 2 mL (when mixed) contains methylprednisolone sodium succinate equivalent to 125 mg methylprednisolone; also |
500 mg Act-O-Vial System (Single-Dose Vial)—Each 4 mL (when mixed) contains methylprednisolone sodium succinate equivalent to 500 mg methylprednisolone; also |
1 gram Act-O-Vial System (Single-Dose Vial)—Each 8 mL (when mixed) contains methylprednisolone sodium succinate equivalent to 1 gram methylprednisolone; also |
500 mg Vial—Each 8 mL (when mixed as directed) contains methylprednisolone sodium succinate equivalent to 500 mg methylprednisolone; also 6.4 mg monobasic sodium phosphate anhydrous; 69.6 mg dibasic sodium phosphate dried. |
1 gram Vial—Each 16 mL (when mixed as directed) contains methylprednisolone sodium succinate equivalent to 1 gram methylprednisolone; also 12.8 mg monobasic sodium phosphate anhydrous; 139.2 mg dibasic sodium phosphate dried. |
2 gram Vial—Each 30.6 mL (when mixed as directed) contains methylprednisolone sodium succinate equivalent to 2 grams methylprednisolone; also 25.6 mg monobasic sodium phosphate anhydrous; 278 mg dibasic sodium phosphate dried. |
2 gram Vial with Diluent—Each 30.6 mL (when mixed as directed) contains methylprednisolone sodium succinate equivalent to 2 grams methylprednisolone; also 25.6 mg monobasic sodium phosphate anhydrous; 278 mg dibasic sodium phosphate dried. |
IMPORTANT — Use only the accompanying diluent |
When necessary, the pH of each formula was adjusted with sodium hydroxide so that the pH of the reconstituted solution is within the USP specified range of 7 to 8.
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.