PREMARIN® VAGINAL CREAM Medication Guide
(conjugated estrogens)
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Patient Information and Instructions for Use).
17.1 Vaginal Bleeding
Inform postmenopausal women of the importance of reporting vaginal bleeding to their healthcare provider as soon as possible [see Warnings and Precautions (5.3)].
17.2 Possible Serious Adverse Reactions with Estrogen-Alone Therapy
Inform postmenopausal women of possible serious adverse reactions of estrogen-alone therapy including Cardiovascular Disorders, Malignant Neoplasms, and Probable Dementia [see Warnings and Precautions (5.2, 5.3, 5.4)].
FDA-Approved Patient Labeling
PREMARIN (prem-uh-rin)
(Conjugated estrogens) Vaginal Cream
Read this PATIENT INFORMATION before you start using PREMARIN Vaginal Cream and read what you get each time you refill your PREMARIN Vaginal Cream prescription. There may be new information. This information does not take the place of talking to your healthcare provider about your menopausal symptoms or your treatment.
What is the most important information I should know about PREMARIN Vaginal Cream (an estrogen mixture)?
- •
- Using estrogen-alone may increase your chance of getting cancer of the uterus (womb) Report any unusual vaginal bleeding right away while you are using PREMARIN Vaginal Cream. Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
- •
- Do not use estrogen-alone to prevent heart disease, heart attacks, strokes or dementia (decline in brain function)
- •
- Using estrogen-alone may increase your chances of getting strokes or blood clots
- •
- Using estrogen-alone may increase your chance of getting dementia, based on a study of women age 65 years of age or older
- •
- Do not use estrogens with progestins to prevent heart disease, heart attacks, strokes or dementia
- •
- Using estrogens with progestins may increase your chances of getting heart attacks, strokes, breast cancer, or blood clots
- •
- Using estrogens with progestins may increase your chance of getting dementia, based on a study of women age 65 years of age or older
- •
- You and your healthcare provider should talk regularly about whether you still need treatment with PREMARIN Vaginal Cream
What is PREMARIN Vaginal Cream?
PREMARIN Vaginal Cream is a medicine that contains a mixture of estrogen hormones.
What is PREMARIN Vaginal Cream used for?
PREMARIN Vaginal Cream is used after menopause to:
- •
- Treat menopausal changes in and around the vagina
You and your healthcare provider should talk regularly about whether you still need treatment with PREMARIN Vaginal Cream to control these problems. - •
- Treat painful intercourse caused by menopausal changes of the vagina
Who should not use PREMARIN Vaginal Cream?
Do not start using PREMARIN Vaginal Cream if you:
- •
- Have unusual vaginal bleeding
- •
- Currently have or have had certain cancers
Estrogens may increase the chance of getting certain types of cancers, including cancer of the breast or uterus. If you have or have had cancer, talk with your healthcare provider about whether you should use PREMARIN Vaginal Cream. - •
- Had a stroke or heart attack
- •
- Currently have or have had blood clots
- •
- Currently have or have had liver problems
- •
- Have been diagnosed with a bleeding disorder
- •
- Are allergic to PREMARIN Vaginal Cream or any of its ingredients
See the list of ingredients in PREMARIN Vaginal Cream at the end of this leaflet. - •
- Think you may be pregnant
Tell your healthcare provider:
- •
- If you have unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause. - •
- About all of your medical problems
Your healthcare provider may need to check you more carefully if you have certain conditions, such as asthma (wheezing), epilepsy (seizures), diabetes, migraine, endometriosis, lupus, or problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood. - •
- About all the medicines you take
This includes prescription and nonprescription medicines, vitamins, and herbal supplements. Some medicines may affect how PREMARIN Vaginal Cream works. PREMARIN Vaginal Cream may also affect how your other medicines work. - •
- If you are going to have surgery or will be on bedrest
You may need to stop using PREMARIN Vaginal Cream. - •
- If you are breast feeding
The estrogen hormones in PREMARIN Vaginal Cream can pass into your breast milk.
How should I use PREMARIN Vaginal Cream?
PREMARIN Vaginal Cream is a cream that you place in your vagina with the applicator provided with the cream.
- •
- Take the dose recommended by your healthcare provider and talk to him or her about how well that dose is working for you
- •
- Estrogens should be used at the lowest dose possible for your treatment only as long as needed. You and your healthcare provider should talk regularly (for example, every 3 to 6 months) about the dose you are taking and whether you still need treatment with PREMARIN Vaginal Cream
- •
- Step 1. Remove cap from tube.
- •
- Step 2. Screw nozzle end of applicator onto tube (Figure A).
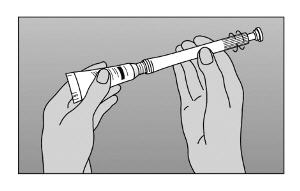 |
Figure A |
- •
- Step 3. Gently squeeze tube from the bottom to force sufficient cream into the barrel to provide the prescribed dose. Use the marked stopping points on the applicator to measure the correct dose, as prescribed by your healthcare provider (Figure B).
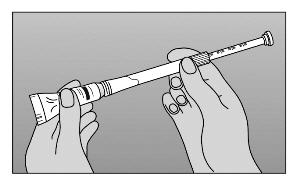 |
Figure B |
- •
- Step 4. Unscrew applicator from tube.
- •
- Step 5. Lie on back with knees drawn up. To deliver medication, gently insert applicator deeply into vagina and press plunger downward to its original position (Figure C).
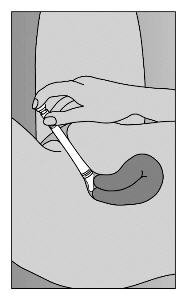 |
Figure C |
- Step 6. TO CLEANSE: Pull plunger to remove it from barrel. Wash with mild soap and warm water (Figure D).
- DO NOT BOIL OR USE HOT WATER.
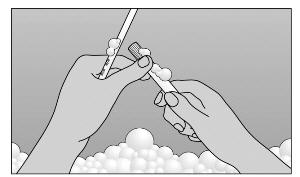 |
Figure D |
What are the possible side effects of PREMARIN Vaginal Cream?
PREMARIN Vaginal Cream is only used in and around the vagina; however, the risks associated with oral estrogens should be taken into account.
Side effects are grouped by how serious they are and how often they happen when you are treated.
Serious, but less common side effects include:
- •
- Heart attack
- •
- Stroke
- •
- Blood clots
- •
- Dementia
- •
- Breast cancer
- •
- Cancer of the lining of the uterus (womb)
- •
- Cancer of the ovary
- •
- High blood pressure
- •
- High blood sugar
- •
- Gallbladder disease
- •
- Liver problems
- •
- Enlargement of benign tumors of the uterus ("fibroids")
- •
- Severe allergic reaction
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- •
- New breast lumps
- •
- Unusual vaginal bleeding
- •
- Changes in vision or speech
- •
- Sudden new severe headaches
- •
- Severe pains in your chest or legs with or without shortness of breath, weakness and fatigue
- •
- Swollen lips, tongue or face
- •
- Less serious, but common side effects include:
- •
- Headache
- •
- Breast pain
- •
- Irregular vaginal bleeding or spotting
- •
- Stomach or abdominal cramps, bloating
- •
- Nausea and vomiting
- •
- Hair loss
- •
- Fluid retention
- •
- Vaginal yeast infection
- •
- Reactions from inserting PREMARIN Vaginal Cream, such as vaginal burning, irritation, and itching
These are not all the possible side effects of PREMARIN Vaginal Cream. For more information, ask your healthcare provider or pharmacist for advice about side effects. You may report side effects to Pfizer Inc. at 1-800-438-1985 or to FDA at 1-800-FDA-1088.
What can I do to lower my chances of getting a serious side effect with PREMARIN Vaginal Cream?
- •
- Talk with your healthcare provider regularly about whether you should continue using PREMARIN Vaginal Cream
- •
- If you have a uterus, talk with your healthcare provider about whether the addition of a progestin is right for you
The addition of a progestin is generally recommended for a woman with a uterus to reduce the chance of getting cancer of the uterus. See your healthcare provider right away if you get vaginal bleeding while using PREMARIN Vaginal Cream. - •
- Have a pelvic exam, breast exam and mammogram (breast X-ray) every year unless your healthcare provider tells you something else
If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram, you may need to have breast exams more often. - •
- If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have higher chances for getting heart disease
Ask your healthcare provider for ways to lower your chances for getting heart disease.
General information about the safe and effective use of PREMARIN Vaginal Cream
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use PREMARIN Vaginal Cream for conditions for which it was not prescribed. Do not give PREMARIN Vaginal Cream to other people, even if they have the same symptoms you have. It may harm them.
Keep PREMARIN Vaginal Cream out of the reach of children.
Latex or rubber condoms, diaphragms and cervical caps may be weakened and fail when they come into contact with PREMARIN Vaginal Cream.
This leaflet provides a summary of the most important information about PREMARIN Vaginal Cream. If you would like more information, talk with your healthcare provider or pharmacist. You can ask for information about PREMARIN Vaginal Cream that is written for health professionals.
What are the ingredients in PREMARIN Vaginal Cream?
PREMARIN Vaginal Cream contains a mixture of conjugated estrogens, which are a mixture of sodium estrone sulfate and sodium equilin sulfate and other components, including sodium sulfate conjugates: 17 α-dihydroequilin, 17 α-estradiol, and 17 β-dihydroequilin. PREMARIN Vaginal Cream also contains cetyl esters wax, cetyl alcohol, white wax, glyceryl monostearate, propylene glycol monostearate, methyl stearate, benzyl alcohol, sodium lauryl sulfate, glycerin, and mineral oil.
PREMARIN (conjugated estrogens) Vaginal Cream—Each gram contains 0.625 mg conjugated estrogens, USP.
Combination package: Each contains a net wt. of 1.06 oz (30 g) tube with plastic applicator(s) calibrated in 0.5 g increments to a maximum of 2 g (NDC 0046-0872-21).
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
This product's label may have been updated. For current full prescribing information, please visit www.pfizer.com.
LAB-0519-7.0
Rev 02/2024
Find PREMARIN® VAGINAL CREAM medical information:
Find PREMARIN® VAGINAL CREAM medical information:
PREMARIN® VAGINAL CREAM Quick Finder
Health Professional Information
Medication Guide
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Patient Information and Instructions for Use).
17.1 Vaginal Bleeding
Inform postmenopausal women of the importance of reporting vaginal bleeding to their healthcare provider as soon as possible [see Warnings and Precautions (5.3)].
17.2 Possible Serious Adverse Reactions with Estrogen-Alone Therapy
Inform postmenopausal women of possible serious adverse reactions of estrogen-alone therapy including Cardiovascular Disorders, Malignant Neoplasms, and Probable Dementia [see Warnings and Precautions (5.2, 5.3, 5.4)].
FDA-Approved Patient Labeling
PREMARIN (prem-uh-rin)
(Conjugated estrogens) Vaginal Cream
Read this PATIENT INFORMATION before you start using PREMARIN Vaginal Cream and read what you get each time you refill your PREMARIN Vaginal Cream prescription. There may be new information. This information does not take the place of talking to your healthcare provider about your menopausal symptoms or your treatment.
What is the most important information I should know about PREMARIN Vaginal Cream (an estrogen mixture)?
- •
- Using estrogen-alone may increase your chance of getting cancer of the uterus (womb) Report any unusual vaginal bleeding right away while you are using PREMARIN Vaginal Cream. Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
- •
- Do not use estrogen-alone to prevent heart disease, heart attacks, strokes or dementia (decline in brain function)
- •
- Using estrogen-alone may increase your chances of getting strokes or blood clots
- •
- Using estrogen-alone may increase your chance of getting dementia, based on a study of women age 65 years of age or older
- •
- Do not use estrogens with progestins to prevent heart disease, heart attacks, strokes or dementia
- •
- Using estrogens with progestins may increase your chances of getting heart attacks, strokes, breast cancer, or blood clots
- •
- Using estrogens with progestins may increase your chance of getting dementia, based on a study of women age 65 years of age or older
- •
- You and your healthcare provider should talk regularly about whether you still need treatment with PREMARIN Vaginal Cream
What is PREMARIN Vaginal Cream?
PREMARIN Vaginal Cream is a medicine that contains a mixture of estrogen hormones.
What is PREMARIN Vaginal Cream used for?
PREMARIN Vaginal Cream is used after menopause to:
- •
- Treat menopausal changes in and around the vagina
You and your healthcare provider should talk regularly about whether you still need treatment with PREMARIN Vaginal Cream to control these problems. - •
- Treat painful intercourse caused by menopausal changes of the vagina
Who should not use PREMARIN Vaginal Cream?
Do not start using PREMARIN Vaginal Cream if you:
- •
- Have unusual vaginal bleeding
- •
- Currently have or have had certain cancers
Estrogens may increase the chance of getting certain types of cancers, including cancer of the breast or uterus. If you have or have had cancer, talk with your healthcare provider about whether you should use PREMARIN Vaginal Cream. - •
- Had a stroke or heart attack
- •
- Currently have or have had blood clots
- •
- Currently have or have had liver problems
- •
- Have been diagnosed with a bleeding disorder
- •
- Are allergic to PREMARIN Vaginal Cream or any of its ingredients
See the list of ingredients in PREMARIN Vaginal Cream at the end of this leaflet. - •
- Think you may be pregnant
Tell your healthcare provider:
- •
- If you have unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause. - •
- About all of your medical problems
Your healthcare provider may need to check you more carefully if you have certain conditions, such as asthma (wheezing), epilepsy (seizures), diabetes, migraine, endometriosis, lupus, or problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood. - •
- About all the medicines you take
This includes prescription and nonprescription medicines, vitamins, and herbal supplements. Some medicines may affect how PREMARIN Vaginal Cream works. PREMARIN Vaginal Cream may also affect how your other medicines work. - •
- If you are going to have surgery or will be on bedrest
You may need to stop using PREMARIN Vaginal Cream. - •
- If you are breast feeding
The estrogen hormones in PREMARIN Vaginal Cream can pass into your breast milk.
How should I use PREMARIN Vaginal Cream?
PREMARIN Vaginal Cream is a cream that you place in your vagina with the applicator provided with the cream.
- •
- Take the dose recommended by your healthcare provider and talk to him or her about how well that dose is working for you
- •
- Estrogens should be used at the lowest dose possible for your treatment only as long as needed. You and your healthcare provider should talk regularly (for example, every 3 to 6 months) about the dose you are taking and whether you still need treatment with PREMARIN Vaginal Cream
- •
- Step 1. Remove cap from tube.
- •
- Step 2. Screw nozzle end of applicator onto tube (Figure A).
 |
Figure A |
- •
- Step 3. Gently squeeze tube from the bottom to force sufficient cream into the barrel to provide the prescribed dose. Use the marked stopping points on the applicator to measure the correct dose, as prescribed by your healthcare provider (Figure B).
 |
Figure B |
- •
- Step 4. Unscrew applicator from tube.
- •
- Step 5. Lie on back with knees drawn up. To deliver medication, gently insert applicator deeply into vagina and press plunger downward to its original position (Figure C).
 |
Figure C |
- Step 6. TO CLEANSE: Pull plunger to remove it from barrel. Wash with mild soap and warm water (Figure D).
- DO NOT BOIL OR USE HOT WATER.
 |
Figure D |
What are the possible side effects of PREMARIN Vaginal Cream?
PREMARIN Vaginal Cream is only used in and around the vagina; however, the risks associated with oral estrogens should be taken into account.
Side effects are grouped by how serious they are and how often they happen when you are treated.
Serious, but less common side effects include:
- •
- Heart attack
- •
- Stroke
- •
- Blood clots
- •
- Dementia
- •
- Breast cancer
- •
- Cancer of the lining of the uterus (womb)
- •
- Cancer of the ovary
- •
- High blood pressure
- •
- High blood sugar
- •
- Gallbladder disease
- •
- Liver problems
- •
- Enlargement of benign tumors of the uterus ("fibroids")
- •
- Severe allergic reaction
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- •
- New breast lumps
- •
- Unusual vaginal bleeding
- •
- Changes in vision or speech
- •
- Sudden new severe headaches
- •
- Severe pains in your chest or legs with or without shortness of breath, weakness and fatigue
- •
- Swollen lips, tongue or face
- •
- Less serious, but common side effects include:
- •
- Headache
- •
- Breast pain
- •
- Irregular vaginal bleeding or spotting
- •
- Stomach or abdominal cramps, bloating
- •
- Nausea and vomiting
- •
- Hair loss
- •
- Fluid retention
- •
- Vaginal yeast infection
- •
- Reactions from inserting PREMARIN Vaginal Cream, such as vaginal burning, irritation, and itching
These are not all the possible side effects of PREMARIN Vaginal Cream. For more information, ask your healthcare provider or pharmacist for advice about side effects. You may report side effects to Pfizer Inc. at 1-800-438-1985 or to FDA at 1-800-FDA-1088.
What can I do to lower my chances of getting a serious side effect with PREMARIN Vaginal Cream?
- •
- Talk with your healthcare provider regularly about whether you should continue using PREMARIN Vaginal Cream
- •
- If you have a uterus, talk with your healthcare provider about whether the addition of a progestin is right for you
The addition of a progestin is generally recommended for a woman with a uterus to reduce the chance of getting cancer of the uterus. See your healthcare provider right away if you get vaginal bleeding while using PREMARIN Vaginal Cream. - •
- Have a pelvic exam, breast exam and mammogram (breast X-ray) every year unless your healthcare provider tells you something else
If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram, you may need to have breast exams more often. - •
- If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have higher chances for getting heart disease
Ask your healthcare provider for ways to lower your chances for getting heart disease.
General information about the safe and effective use of PREMARIN Vaginal Cream
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use PREMARIN Vaginal Cream for conditions for which it was not prescribed. Do not give PREMARIN Vaginal Cream to other people, even if they have the same symptoms you have. It may harm them.
Keep PREMARIN Vaginal Cream out of the reach of children.
Latex or rubber condoms, diaphragms and cervical caps may be weakened and fail when they come into contact with PREMARIN Vaginal Cream.
This leaflet provides a summary of the most important information about PREMARIN Vaginal Cream. If you would like more information, talk with your healthcare provider or pharmacist. You can ask for information about PREMARIN Vaginal Cream that is written for health professionals.
What are the ingredients in PREMARIN Vaginal Cream?
PREMARIN Vaginal Cream contains a mixture of conjugated estrogens, which are a mixture of sodium estrone sulfate and sodium equilin sulfate and other components, including sodium sulfate conjugates: 17 α-dihydroequilin, 17 α-estradiol, and 17 β-dihydroequilin. PREMARIN Vaginal Cream also contains cetyl esters wax, cetyl alcohol, white wax, glyceryl monostearate, propylene glycol monostearate, methyl stearate, benzyl alcohol, sodium lauryl sulfate, glycerin, and mineral oil.
PREMARIN (conjugated estrogens) Vaginal Cream—Each gram contains 0.625 mg conjugated estrogens, USP.
Combination package: Each contains a net wt. of 1.06 oz (30 g) tube with plastic applicator(s) calibrated in 0.5 g increments to a maximum of 2 g (NDC 0046-0872-21).
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
This product's label may have been updated. For current full prescribing information, please visit www.pfizer.com.
LAB-0519-7.0
Rev 02/2024
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.
