DESCRIPTION
Buffered Pfizerpen (Penicillin G Potassium for Injection, USP) is a sterile, pyrogen-free powder for reconstitution. Buffered Pfizerpen for Injection is an antibacterial agent for intramuscular, continuous intravenous infusion, intrapleural or other local infusion, and intrathecal administration.
Each million units contains approximately 6.8 milligrams of sodium (0.3 mEq) and 65.6 milligrams of potassium (1.68 mEq). Buffered Pfizerpen (Penicillin G Potassium for Injection, USP) is supplied in vials equivalent to 5,000,000 units (5 million units) or 20,000,000 units (20 million units) of penicillin G as the potassium salt.
Chemically, Pfizerpen is monopotassium 3,3-dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo (3.2.0) heptane-2-carboxylate. It has a molecular weight of 372.48 and the following chemical structure:
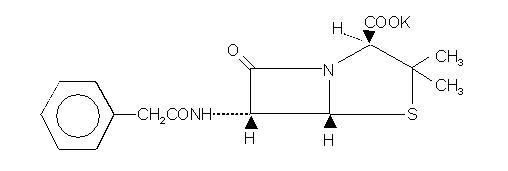 |
Formula
C16H17KN2O4S |
Penicillin G potassium is a colorless or white crystal, or a white crystalline powder which is odorless, or practically so, and moderately hygroscopic. Penicillin G potassium is very soluble in water. The pH of the reconstituted product is between 6.0–8.5.