ZAVZPRET™
([zavegepant] nasal spray)
Find ZAVZPRET™ medical information:
Find ZAVZPRET™ medical information:
ZAVZPRET™ Quick Finder
Patient Package Insert
Patient Package Insert
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Hypersensitivity Reactions
Inform patients about the signs and symptoms of hypersensitivity reactions after administration of ZAVZPRET. Advise patients to contact their healthcare provider immediately if signs or symptoms of hypersensitivity reactions occur [see Warnings and Precautions (5.1)].
Drug Interactions
Advise patients to speak with their healthcare provider about any prescription or over-the-counter medications or herbal supplements that they take or plan to take. Inform patients that if they need to use an intranasal decongestant it should be administered at least 1 hour after ZAVZPRET administration [see Drug Interactions (7.3)].
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
LAB-1544-1.0
Patient Package Insert
This Patient Information has been approved by the US. Food and Drug Administration | 3/2023 |
PATIENT INFORMATION (zavegepant) nasal spray | |
What is ZAVZPRET? ZAVZPRET is a prescription medicine used in adults for the acute treatment of migraine attacks with or without aura. ZAVZPRET is not used to prevent migraine attacks. It is not known if ZAVZPRET is safe and effective in children. | |
Do not use ZAVZPRET if you are:
See the end of this leaflet for a complete list of ingredients in ZAVZPRET. | |
Before you use ZAVZPRET, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | |
How should I use ZAVZPRET?
| |
What are the possible side effects of ZAVZPRET? ZAVZPRET may cause serious side effects including:
The most common side effects of ZAVZPRET are:
These are not the only possible side effects of ZAVZPRET. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1 800-FDA-1088. | |
How should I store ZAVZPRET?
Keep ZAVZPRET and all medicines out of the reach of children. | |
General information about the safe and effective use of ZAVZPRET: Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ZAVZPRET for a condition for which it was not prescribed. Do not give ZAVZPRET to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZAVZPRET that is written for health professionals. | |
What are the ingredients in ZAVZPRET? Active ingredients in ZAVZPRET: zavegepant Inactive ingredients in ZAVZPRET: dextrose, hydrochloric acid, sodium hydroxide, and succinic acid in water for injection.  For more information, go to www.zavzpret.com or call 1-800-438-1985. LAB-1545-1.0 | |
Instructions for Use
INSTRUCTIONS FOR USE ZAVZPRET [zav-spret] (zavegepant) nasal spray For Nasal Use Only | ||
This Instructions for Use contains information on how to give a single dose (10 mg) with ZAVZPRET nasal spray One (1) device delivers a single dose of ZAVZPRET. The device provides 1 spray that should be delivered into 1 nostril. | ||
ZAVZPRET Device Parts  | ||
Important Information You Need to Know Before Dosing with ZAVZPRET
Storage Information
Preparing to Dose with ZAVZPRET Nasal Spray | ||
1 | Gently Blow Your Nose | |
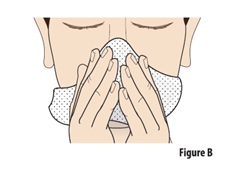
1.1 | Before using ZAVZPRET, blow your nose to clear your nostrils (see Figure B). This can be done while sitting or standing. | |
 | ||
2 | Remove 1 Blister from the Carton | |
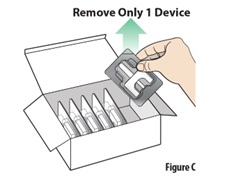
2.1 | Remove 1 blister containing a device from the carton (see Figure C). | |
 | ||
3 | Check Expiration Date on the Blister | |
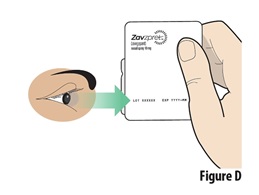
3.1 | Check the expiration date on the blister containing a device (see Figure D). | |
Do not use if the expiration date has passed. Throw away the expired device in the trash if the expiration date has passed. | ||
 | ||
4 | Remove Device from Blister | |
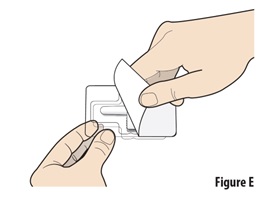
4.1 | Peel open the blister seal from the corner and remove it completely (see Figure E). | |
4.2 | Carefully remove the device from the plastic tray. | |
 | ||
Dosing with ZAVZPRET Nasal Spray | ||
5 | Check Expiration Date on the Device | |
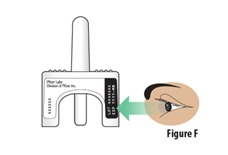
5.1 | Check the expiration date on the device (see Figure F). Do not use if the expiration date has passed. Throw away the expired device in the trash if the expiration date has passed. | |
 | ||
6 | Grip the Device | |
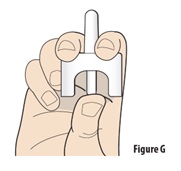
6.1 | Hold the device upright with your thumb on the bottom of the plunger and two fingers on either side of the nozzle (see Figure G). Do not press the plunger yet. If you press the plunger now, the medicine will spray and be wasted. | |
 | ||
Dosing with ZAVZPRET Nasal Spray (Continued) | ||
7 | Close 1 Nostril | |
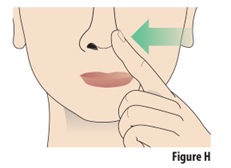
7.1 | With your free hand, gently press 1 nostril to close it (see Figure H). | |
7.2 | Continue breathing normally through your mouth. | |
 | ||
8 | Insert Spray Nozzle Into Open Nostril | |
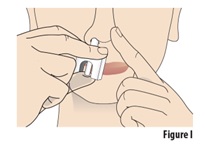
8.1 | Insert the nozzle into the open nostril as much as you comfortably can (see Figure I). | |
 | ||
9 | Deliver the Dose by Spraying Into Nostril and Breathing In | |
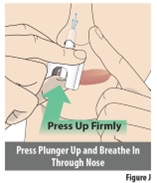
9.1 | Keep your head level and upright and close your mouth. Do not tilt your head. Do not lay down while delivering the dose. | |
9.2 | Slowly breathe in through your nose as you firmly press the plunger up with your thumb to release the spray (see Figure J). Important: Hold the nozzle firmly in your nose as you deliver the dose. Do not let the nozzle come out when pressing the plunger. Spray only 1 time into 1 nostril. | |
 | ||
10 | Remove the Device and Keep Your Head Level for 20 Seconds | |
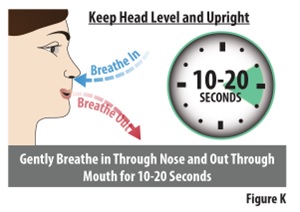
10.1 | Remove the device from your nostril and your finger from your other nostril. | |
10.2 | While keeping your head level and upright, gently breathe in through your nose and out through your mouth for 10 to 20 seconds (see Figure K). | |
If you feel a drip from your nose, gently sniff so you do not lose any of your dose. | ||
 | ||
Throwing away (Disposing of) Device | ||
11 | Throw Away Used Device | |
11.1 | Throw away the used device into trash (see Figure L) | |
 | ||
You have received your full dose, if you have:
Additional Information For more information on ZAVZPRET (zavegepant), visit www.zavzpret.com or call 1-800-438-1985. This Instructions for Use has been approved by the U.S. Food and Drug Administration Approved: 3/2023  LAB-1546-1.0 | ||
What is ZAVZPRET?
What is ZAVZPRET?
ZAVZPRET is a prescription medicine used in adults for the acute treatment of migraine attacks with or without aura.
ZAVZPRET is not used to prevent migraine attacks.
It is not known if ZAVZPRET is safe and effective in children.
Do not use ZAVZPRET if you are:
- •
- allergic to zavegepant, or any of the ingredients in ZAVZPRET.
See the end of this leaflet for a complete list of ingredients in ZAVZPRET.
Before you use ZAVZPRET, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have kidney problems.
- •
- have liver problems.
- •
- are pregnant or plan to become pregnant. It is not known if ZAVZPRET will harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known whether ZAVZPRET passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby if you use ZAVZPRET.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use ZAVZPRET?
How should I use ZAVZPRET?
- •
- Use ZAVZPRET exactly how your healthcare provider tells you to use it.
- •
- See the Instructions for Use for complete information on how to use ZAVZPRET nasal spray.
- •
- ZAVZPRET is given in the nose (nasal) only.
- •
- Each ZAVZPRET only sprays 1 time and cannot be reused. Do not test or prime the nasal spray before use.
- •
- Each dose of ZAVZPRET is provided in an individual pack. Use all of the medicine in 1 pack for a complete dose.
- •
- The recommended dose is 10 mg given as a single spray in one nostril.
- •
- Do not use more than 1 spray (10 mg) of ZAVZPRET nasal spray in a 24-hour period.
- •
- It is not known if it is safe to use more than 8 sprays (doses) of ZAVZPRET in 30 days.
- •
- Avoid using intranasal decongestants with ZAVZPRET. If you have to use an intranasal decongestant, use it at least 1 hour after using ZAVZPRET.
What are the possible side effects of ZAVZPRET?
What are the possible side effects of ZAVZPRET?
ZAVZPRET may cause serious side effects including:
- •
- Allergic reactions. Allergic reactions, including hives and swelling of the face, can happen after you use ZAVZPRET. Call your healthcare provider or get emergency help right away if you have any of the following symptoms, which may be part of an allergic reaction:
- o
- swelling of the face, mouth, tongue, or throat
- o
- trouble breathing
The most common side effects of ZAVZPRET are:
- •
- unusual taste
- •
- nausea
- •
- nasal discomfort
- •
- vomiting
These are not the only possible side effects of ZAVZPRET.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1 800-FDA-1088.
How should I store ZAVZPRET?
How should I store ZAVZPRET?
- •
- Store ZAVZPRET in the blister package that it comes in.
- •
- Store ZAVZPRET at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Do not freeze.
Keep ZAVZPRET and all medicines out of the reach of children.
General information about the safe and effective use of ZAVZPRET:
General information about the safe and effective use of ZAVZPRET:
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ZAVZPRET for a condition for which it was not prescribed. Do not give ZAVZPRET to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZAVZPRET that is written for health professionals.
What are the ingredients in ZAVZPRET?
What are the ingredients in ZAVZPRET?
Active ingredients in ZAVZPRET: zavegepant
Inactive ingredients in ZAVZPRET: dextrose, hydrochloric acid, sodium hydroxide, and succinic acid in water for injection.

For more information, go to www.zavzpret.com or call 1-800-438-1985.
LAB-1545-1.0
Instructions for Use
Instructions for Use
INSTRUCTIONS FOR USE ZAVZPRET [zav-spret] (zavegepant) nasal spray For Nasal Use Only | ||
This Instructions for Use contains information on how to give a single dose (10 mg) with ZAVZPRET nasal spray One (1) device delivers a single dose of ZAVZPRET. The device provides 1 spray that should be delivered into 1 nostril. | ||
ZAVZPRET Device Parts  | ||
Important Information You Need to Know Before Dosing with ZAVZPRET
Storage Information
Preparing to Dose with ZAVZPRET Nasal Spray | ||
1 | Gently Blow Your Nose | |
1.1 | Before using ZAVZPRET, blow your nose to clear your nostrils (see Figure B). This can be done while sitting or standing. | |
 | ||
2 | Remove 1 Blister from the Carton | |
2.1 | Remove 1 blister containing a device from the carton (see Figure C). | |
 | ||
3 | Check Expiration Date on the Blister | |
3.1 | Check the expiration date on the blister containing a device (see Figure D). | |
Do not use if the expiration date has passed. Throw away the expired device in the trash if the expiration date has passed. | ||
 | ||
4 | Remove Device from Blister | |
4.1 | Peel open the blister seal from the corner and remove it completely (see Figure E). | |
4.2 | Carefully remove the device from the plastic tray. | |
 | ||
Dosing with ZAVZPRET Nasal Spray | ||
5 | Check Expiration Date on the Device | |
5.1 | Check the expiration date on the device (see Figure F). Do not use if the expiration date has passed. Throw away the expired device in the trash if the expiration date has passed. | |
 | ||
6 | Grip the Device | |
6.1 | Hold the device upright with your thumb on the bottom of the plunger and two fingers on either side of the nozzle (see Figure G). Do not press the plunger yet. If you press the plunger now, the medicine will spray and be wasted. | |
 | ||
Dosing with ZAVZPRET Nasal Spray (Continued) | ||
7 | Close 1 Nostril | |
7.1 | With your free hand, gently press 1 nostril to close it (see Figure H). | |
7.2 | Continue breathing normally through your mouth. | |
 | ||
8 | Insert Spray Nozzle Into Open Nostril | |
8.1 | Insert the nozzle into the open nostril as much as you comfortably can (see Figure I). | |
 | ||
9 | Deliver the Dose by Spraying Into Nostril and Breathing In | |
9.1 | Keep your head level and upright and close your mouth. Do not tilt your head. Do not lay down while delivering the dose. | |
9.2 | Slowly breathe in through your nose as you firmly press the plunger up with your thumb to release the spray (see Figure J). Important: Hold the nozzle firmly in your nose as you deliver the dose. Do not let the nozzle come out when pressing the plunger. Spray only 1 time into 1 nostril. | |
 | ||
10 | Remove the Device and Keep Your Head Level for 20 Seconds | |
10.1 | Remove the device from your nostril and your finger from your other nostril. | |
10.2 | While keeping your head level and upright, gently breathe in through your nose and out through your mouth for 10 to 20 seconds (see Figure K). | |
If you feel a drip from your nose, gently sniff so you do not lose any of your dose. | ||
 | ||
Throwing away (Disposing of) Device | ||
11 | Throw Away Used Device | |
11.1 | Throw away the used device into trash (see Figure L) | |
 | ||
You have received your full dose, if you have:
Additional Information For more information on ZAVZPRET (zavegepant), visit www.zavzpret.com or call 1-800-438-1985. This Instructions for Use has been approved by the U.S. Food and Drug Administration Approved: 3/2023  LAB-1546-1.0 | ||
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Hypersensitivity Reactions
Inform patients about the signs and symptoms of hypersensitivity reactions after administration of ZAVZPRET. Advise patients to contact their healthcare provider immediately if signs or symptoms of hypersensitivity reactions occur [see Warnings and Precautions (5.1)].
Drug Interactions
Advise patients to speak with their healthcare provider about any prescription or over-the-counter medications or herbal supplements that they take or plan to take. Inform patients that if they need to use an intranasal decongestant it should be administered at least 1 hour after ZAVZPRET administration [see Drug Interactions (7.3)].
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
LAB-1544-1.0
Patient Package Insert
This Patient Information has been approved by the US. Food and Drug Administration | 3/2023 |
PATIENT INFORMATION (zavegepant) nasal spray | |
What is ZAVZPRET? ZAVZPRET is a prescription medicine used in adults for the acute treatment of migraine attacks with or without aura. ZAVZPRET is not used to prevent migraine attacks. It is not known if ZAVZPRET is safe and effective in children. | |
Do not use ZAVZPRET if you are:
See the end of this leaflet for a complete list of ingredients in ZAVZPRET. | |
Before you use ZAVZPRET, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | |
How should I use ZAVZPRET?
| |
What are the possible side effects of ZAVZPRET? ZAVZPRET may cause serious side effects including:
The most common side effects of ZAVZPRET are:
These are not the only possible side effects of ZAVZPRET. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1 800-FDA-1088. | |
How should I store ZAVZPRET?
Keep ZAVZPRET and all medicines out of the reach of children. | |
General information about the safe and effective use of ZAVZPRET: Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ZAVZPRET for a condition for which it was not prescribed. Do not give ZAVZPRET to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZAVZPRET that is written for health professionals. | |
What are the ingredients in ZAVZPRET? Active ingredients in ZAVZPRET: zavegepant Inactive ingredients in ZAVZPRET: dextrose, hydrochloric acid, sodium hydroxide, and succinic acid in water for injection.  For more information, go to www.zavzpret.com or call 1-800-438-1985. LAB-1545-1.0 | |
Instructions for Use
INSTRUCTIONS FOR USE ZAVZPRET [zav-spret] (zavegepant) nasal spray For Nasal Use Only | ||
This Instructions for Use contains information on how to give a single dose (10 mg) with ZAVZPRET nasal spray One (1) device delivers a single dose of ZAVZPRET. The device provides 1 spray that should be delivered into 1 nostril. | ||
ZAVZPRET Device Parts  | ||
Important Information You Need to Know Before Dosing with ZAVZPRET
Storage Information
Preparing to Dose with ZAVZPRET Nasal Spray | ||
1 | Gently Blow Your Nose | |
1.1 | Before using ZAVZPRET, blow your nose to clear your nostrils (see Figure B). This can be done while sitting or standing. | |
 | ||
2 | Remove 1 Blister from the Carton | |
2.1 | Remove 1 blister containing a device from the carton (see Figure C). | |
 | ||
3 | Check Expiration Date on the Blister | |
3.1 | Check the expiration date on the blister containing a device (see Figure D). | |
Do not use if the expiration date has passed. Throw away the expired device in the trash if the expiration date has passed. | ||
 | ||
4 | Remove Device from Blister | |
4.1 | Peel open the blister seal from the corner and remove it completely (see Figure E). | |
4.2 | Carefully remove the device from the plastic tray. | |
 | ||
Dosing with ZAVZPRET Nasal Spray | ||
5 | Check Expiration Date on the Device | |
5.1 | Check the expiration date on the device (see Figure F). Do not use if the expiration date has passed. Throw away the expired device in the trash if the expiration date has passed. | |
 | ||
6 | Grip the Device | |
6.1 | Hold the device upright with your thumb on the bottom of the plunger and two fingers on either side of the nozzle (see Figure G). Do not press the plunger yet. If you press the plunger now, the medicine will spray and be wasted. | |
 | ||
Dosing with ZAVZPRET Nasal Spray (Continued) | ||
7 | Close 1 Nostril | |
7.1 | With your free hand, gently press 1 nostril to close it (see Figure H). | |
7.2 | Continue breathing normally through your mouth. | |
 | ||
8 | Insert Spray Nozzle Into Open Nostril | |
8.1 | Insert the nozzle into the open nostril as much as you comfortably can (see Figure I). | |
 | ||
9 | Deliver the Dose by Spraying Into Nostril and Breathing In | |
9.1 | Keep your head level and upright and close your mouth. Do not tilt your head. Do not lay down while delivering the dose. | |
9.2 | Slowly breathe in through your nose as you firmly press the plunger up with your thumb to release the spray (see Figure J). Important: Hold the nozzle firmly in your nose as you deliver the dose. Do not let the nozzle come out when pressing the plunger. Spray only 1 time into 1 nostril. | |
 | ||
10 | Remove the Device and Keep Your Head Level for 20 Seconds | |
10.1 | Remove the device from your nostril and your finger from your other nostril. | |
10.2 | While keeping your head level and upright, gently breathe in through your nose and out through your mouth for 10 to 20 seconds (see Figure K). | |
If you feel a drip from your nose, gently sniff so you do not lose any of your dose. | ||
 | ||
Throwing away (Disposing of) Device | ||
11 | Throw Away Used Device | |
11.1 | Throw away the used device into trash (see Figure L) | |
 | ||
You have received your full dose, if you have:
Additional Information For more information on ZAVZPRET (zavegepant), visit www.zavzpret.com or call 1-800-438-1985. This Instructions for Use has been approved by the U.S. Food and Drug Administration Approved: 3/2023  LAB-1546-1.0 | ||
Highlights
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZAVZPRET safely and effectively. See full prescribing information for ZAVZPRET. ZAVZPRET™ (zavegepant) nasal spray Initial U.S. Approval: 2023 INDICATIONS AND USAGEDOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSNasal spray: 10 mg (3) CONTRAINDICATIONSPatients with a history of hypersensitivity reaction to zavegepant or to any of the components of ZAVZPRET. (4) WARNINGS AND PRECAUTIONSHypersensitivity Reactions: If a serious hypersensitivity reaction occurs, discontinue ZAVZPRET and initiate appropriate therapy. Hypersensitivity Reactions including facial swelling and urticaria have occurred with ZAVZPRET. (5.1) ADVERSE REACTIONSMost common adverse reactions (at least 2% of patients treated with ZAVZPRET and greater than placebo) were taste disorders, nausea, nasal discomfort, and vomiting. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 3/2023 |
Indications and Usage
Dosage and Administration
Dosage Forms and Strengths
Contraindications
4 CONTRAINDICATIONS
ZAVZPRET is contraindicated in patients with a history of hypersensitivity reaction to zavegepant or any of the components of ZAVZPRET [see Warnings and Precautions (5.1)].
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including facial swelling and urticaria, have occurred in patients treated with ZAVZPRET in clinical studies. If a hypersensitivity reaction occurs, discontinue ZAVZPRET and initiate appropriate therapy [see Contraindications (4) and Adverse Reactions (6.1)].
Adverse Reactions
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of ZAVZPRET for the acute treatment of migraine in adults has been evaluated in two randomized, double-blind, placebo-controlled trials (Study 1 and Study 2) in patients with migraine who received one 10 mg dose of ZAVZPRET nasal spray (N=1023) or placebo (N=1056) [see Clinical Studies (14)]. Approximately 83% were female, 81% were White, 20% were Hispanic or Latino, and 15% were Black. The mean age at study entry was 41 years (range 18-79 years of age).
Adverse reactions in Study 1 and 2 are shown in Table 1.
Table 1: Adverse Reactions Occurring in At Least 2% of Patients Treated with ZAVZPRET and at a Frequency Greater than Placebo in Study 1 and 2
| ||
Adverse Reaction | ZAVZPRET N=1023 % | Placebo N=1056 % |
Taste Disorders* | 18 | 4 |
Nausea | 4 | 1 |
Nasal Discomfort | 3 | 1 |
Vomiting | 2 | <1 |
Hypersensitivity, including facial swelling and urticaria, occurred in less than 1% of patients treated with ZAVZPRET [see Contraindications (4) and Warnings and Precautions (5.1)].
Long-term safety was assessed in an open-label extension study. That study evaluated 603 patients, dosing intermittently for up to one year, including 360 patients who were exposed to ZAVZPRET 10 mg for at least 6 months, and 298 who were exposed for at least one year, all of whom treated an average of at least two migraine attacks per month.
Drug Interactions
7 DRUG INTERACTIONS
7.1 OATP1B3 or NTCP Inhibitors
Concomitant administration of ZAVZPRET with inhibitors of the organic anion transporting polypeptide 1B3 (OATP1B3) or sodium taurocholate co-transporting polypeptide (NTCP) transporters may result in a significant increase in zavegepant exposure. Avoid concomitant administration of ZAVZPRET with drugs that inhibit OATP1B3 or NTCP transporters [see Clinical Pharmacology (12.3)].
7.2 OATP1B3 or NTCP Inducers
Concomitant administration of ZAVZPRET with inducers of OATP1B3 or NTCP transporters may result in a decrease in zavegepant exposure. Avoid concomitant administration of ZAVZPRET with drugs that induce OATP1B3 or NTCP transporters [see Clinical Pharmacology (12.3)].
7.3 Intranasal Decongestants
Concomitant administration of ZAVZPRET with intranasal decongestants may decrease the absorption of zavegepant. Avoid concomitant administration of intranasal decongestants with ZAVZPRET. When concomitant use is unavoidable, intranasal decongestants should be administered at least 1 hour after ZAVZPRET administration [see Clinical Pharmacology (12.3)].
Use in Specific Populations
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with the use of ZAVZPRET in pregnant women. No adverse developmental effects were observed following subcutaneous administration of zavegepant to pregnant animals at doses associated with plasma exposures higher than those used clinically (see Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. The estimated rate of major birth defects (2.2 to 2.9%) and miscarriage (17%) among deliveries to women with migraine are similar to rates reported in women without migraine.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Published data have suggested that women with migraine may be at increased risk of preeclampsia and gestational hypertension during pregnancy.
Animal Data
Subcutaneous administration of zavegepant to pregnant rats (0, 10, 20, or 40 mg/kg/day) or rabbits (0, 20, 40, or 60 mg/kg/day) during the period of organogenesis resulted in no adverse effects on embryofetal development. Plasma exposures (AUC) at the highest doses tested were approximately 4000 times that in humans at the maximum recommended human dose (MRHD) of 10 mg/day.
Subcutaneous administration of zavegepant (0, 5, 10, or 20 mg/kg/day) to rats throughout pregnancy and lactation resulted in no adverse effects on pre- and postnatal development. Plasma exposure (AUC) at the highest dose tested was approximately 2500 times that in humans at the MRHD.
8.2 Lactation
There are no data on the presence of zavegepant or its metabolites in human milk, the effects of zavegepant on the breastfed infant, or the effects of zavegepant on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ZAVZPRET and any potential adverse effects on the breastfed infant from ZAVZPRET or from the underlying maternal condition.
8.5 Geriatric Use
Clinical studies of ZAVZPRET did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
In a limited number of patients 65 years of age and older, no clinically significant pharmacokinetic differences were observed between elderly and younger subjects.
8.6 Hepatic Impairment
No dosage adjustment of ZAVZPRET is necessary in patients with mild hepatic impairment (Child-Pugh Class A) or moderate hepatic impairment (Child-Pugh Class B). ZAVZPRET has not been studied in patients with severe hepatic impairment (Child-Pugh Class C). Avoid use of ZAVZPRET in patients with severe hepatic impairment [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dosage adjustment of ZAVZPRET is necessary in patients with estimated creatine clearance (CLcr) 30 mL/min or greater. Avoid use of ZAVZPRET in patients with CLcr less than 30 mL/min [see Clinical Pharmacology (12.3)].
Description
11 DESCRIPTION
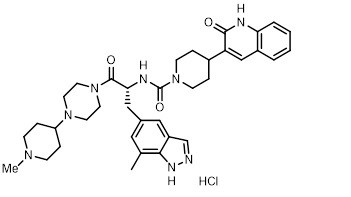
ZAVZPRET (zavegepant) nasal spray contains zavegepant hydrochloride, a calcitonin gene-related peptide receptor antagonist. Zavegepant hydrochloride is described chemically as (R)-N-(3-(7-methyl-1H-indazol-5-yl)-1-(4-(1-methylpiperidin-4-yl) piperazin-1-yl)-1-oxopropan-2-yl)-4-(2-oxo-1,2-dihydroquinolin-3-yl) piperidine-1-carboxamide hydrochloride and its structural formula is:
Its molecular formula is C36H46N8O3․HCl, representing a molecular weight of 675.28 g/mol. Zavegepant free base has a molecular weight of 638.82 g/mol. Zavegepant hydrochloride is a white to off-white powder, freely soluble in water, and has pKa values of 4.8 and 8.8.
Each unit-dose ZAVZPRET device for nasal administration delivers 10 mg of zavegepant (equivalent to 10.6 mg of zavegepant hydrochloride) in a buffered aqueous solution containing dextrose, hydrochloric acid, sodium hydroxide, and succinic acid in water for injection. The solution has a pH of 5.3 to 6.7.
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
The relationship between pharmacodynamic activity and the mechanism by which zavegepant exerts its clinical effects is unknown.
No clinically relevant differences in resting blood pressure were observed when zavegepant was concomitantly administered with sumatriptan (12 mg subcutaneous, given as two 6 mg doses separated by one hour) compared with sumatriptan alone to healthy volunteers.
Cardiac Electrophysiology
At a dose up to 4 times the recommended daily dose, zavegepant does not prolong the QT interval to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption
Peak plasma concentration of zavegepant was observed at approximately 30 minutes after a single 10 mg dose of the nasal spray. After nasal spray administration of zavegepant, the absolute bioavailability is approximately 5%.
Zavegepant given as a single dose of the nasal spray displays slightly less than dose-proportional pharmacokinetics up to 40 mg (approximately 4 times the recommended dosage of 10 mg).
Following once daily dosing of ZAVZPRET for 14 days there was no evidence of zavegepant accumulation.
Distribution
The mean apparent volume of distribution of intranasal zavegepant is approximately 1774 L. Plasma protein binding of zavegepant is approximately 90%.
Elimination
Metabolism
Zavegepant is primarily metabolized by CYP3A4 and to a lesser extent by CYP2D6, in vitro. After single IV dose of 5 mg [14C]-zavegepant, unchanged zavegepant was the most prevalent (approximately 90%) circulating component in the human plasma. No major metabolites (i.e., greater than 10%) of zavegepant were detected in plasma.
Excretion
The effective half-life of zavegepant following a 10 mg dose of the nasal spray is 6.55 hours. The mean apparent clearance of intranasal zavegepant is 266 L/h. Zavegepant is excreted mostly via the biliary/fecal route, while the renal route is a minor route of elimination. Following a single intravenous dose of 5 mg [14C]-zavegepant to healthy male subjects, approximately 80% and 11% of the dose was recovered as unchanged zavegepant in feces and urine, respectively.
Specific Populations
Patients with Hepatic Impairment
In a dedicated clinical study comparing the pharmacokinetics of zavegepant in subjects with moderate hepatic impairment (Child-Pugh B) to that of normal subjects (matched healthy controls), zavegepant Cmax was 16% higher and AUC was 1.9-fold higher in patients with moderate hepatic impairment. These changes in exposures are not expected to be clinically significant, based on clinical safety experience and minimal accumulation of drug exposures. The impact of severe hepatic impairment (Child-Pugh C) on the pharmacokinetics of zavegepant was not studied [see Use in Specific Populations (8.6)].
Patients with Renal Impairment
The renal route plays a minor role in the clearance of zavegepant. No clinically significant effect on the pharmacokinetics of zavegepant is expected in subjects with estimated creatinine clearance (CLcr) 30 mL/min or greater. In patients with CLcr 15 to 29 mL/min, accumulation of uremic solutes can cause an increase in zavegepant exposures by inhibiting OATP transporters. Zavegepant has not been studied in patients with CLcr less than 15 mL/min [see Use in Specific Populations (8.7)].
Other Specific Populations
Age, sex, race, ethnicity, and body weight did not show clinically significant effects on the pharmacokinetics of zavegepant.
Drug Interaction Studies
In Vitro Studies
Enzymes
Zavegepant is a substrate of CYP3A4 and to a lesser extent CYP2D6. Zavegepant is not an inducer of CYP1A2, 2B6, or 3A4, or an inhibitor of CYP1A2, CYP2C9, 2C19, 2B6, 2D6, 2C8, and 3A4 at clinically relevant concentrations.
Transporters
Zavegepant is a substrate for OATP1B3 and NTCP (see In Vivo studies).
Zavegepant is also a substrate for the transporters P-gp, MATE1, and MATE2-K. Considering the minor contribution of the renal pathway in the clearance of zavegepant, coadministration of zavegepant with inhibitors of P-gp, MATE1, and MATE2-K inhibitors is not expected to result in a clinically significant effect on zavegepant pharmacokinetics.
Zavegepant is not a substrate for BCRP, OATP1B1, OAT1, OAT3, OCT2, BSEP, MRP2, MRP3, and MRP4.
Zavegepant is an inhibitor of OCT2, MATE1, and MATE2-K, but drug interactions for ZAVZPRET are not expected at clinically relevant concentrations. Zavegepant is not an inhibitor of P-gp, BCRP, OAT1, OAT3, OATP1B1, and OATP1B3.
CYP3A4 Inhibitors
Concomitant administration of a single dose of 10 mg ZAVZPRET with itraconazole (a strong CYP3A4 and P-gp inhibitor), at steady state did not result in a clinically relevant effect on the exposures of zavegepant.
OATP1B3 or NTCP Inhibitors
Concomitant administration of a single oral dose of 100 mg zavegepant with rifampin (an OATP1B3, NTCP inhibitor and a strong CYP3A inducer), at steady state resulted in increased zavegepant exposure (AUC by 2.3-fold and Cmax by 2.2-fold). The observed change in zavegepant exposures is a composite effect of inhibition of OATP1B3 and NTCP transporters as well as induction of CYP3A enzymes. Concomitant administration of ZAVZPRET with inhibitors of OATP1B3 or NTCP transporters may result in a significant increase in zavegepant exposure [see Drug Interactions (7.1)].
OATP1B3 or NTCP Inducers
Concomitant administration of ZAVZPRET with inducers of OATP1B3 or NTCP transporters has not been studied. However, since zavegepant is a substrate of OATP1B3 and NTCP, concomitant administration with inducers of these transporters may result in decreased zavegepant exposure [see Drug Interactions (7.2)].
Intranasal Decongestants
The effect of concomitant intranasal decongestants on the pharmacokinetics of zavegepant nasal spray has not been evaluated. Concomitant administration of intranasal decongestants may decrease the systemic exposure of zavegepant and potentially the efficacy of zavegepant [see Drug Interactions (7.3)].
Other Drugs
No significant pharmacokinetic interactions were observed when zavegepant was concomitantly administered with oral contraceptives (ethinyl estradiol) or sumatriptan [see Clinical Pharmacology (12.2)].
Nonclinical Toxicology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Intranasal administration of zavegepant (0, 0.3, 0.8, or 2.5 mg/day) to Tg.rasH2 mice for 26 weeks resulted in no evidence of drug-related tumors.
Intranasal administration of zavegepant (0, 2, 9, or 18.8 mg/kg/day) to rats for up to 96 weeks resulted in no evidence of drug-related tumors. Plasma exposure (AUC) at the highest dose tested was approximately 140 times that in humans at the maximum recommended human dose (MRHD) of 10 mg/day.
Mutagenesis
Zavegepant was negative in in vitro (bacterial reverse mutation, chromosomal aberration in Chinese hamster ovary cells) and in vivo (rat micronucleus) assays.
Impairment of Fertility
Subcutaneous administration of zavegepant (0, 5, 15, or 25 mg/kg/day) to male and female rats prior to and during mating and continuing in females to gestation day 7 resulted in no adverse effects on fertility or reproductive performance. Plasma exposures (AUC) at the highest dose tested were approximately 2800 times that in humans at MRHD.
Clinical Studies
14 CLINICAL STUDIES
The efficacy of ZAVZPRET for the acute treatment of migraine with or without aura in adults was demonstrated in two randomized, double-blind, placebo-controlled trials (Study 1 and Study 2). In both studies, patients were instructed to treat a migraine of moderate to severe headache pain intensity. Rescue medication (i.e., NSAIDs, acetaminophen, and/or an antiemetic) was allowed 2 hours after the initial treatment. Other forms of rescue medication such as triptans were not allowed within 48 hours of initial treatment. In Study 1 and Study 2, 13.4% and 13.6% of patients were taking preventive medications for migraine at baseline, respectively. None of the patients were on concomitant preventive medication that act on the CGRP pathway.
In Study 1 (NCT04571060), patients were randomized to receive a single dose of ZAVZPRET 10 mg (N=623) or placebo (N=646). Efficacy was demonstrated with ZAVZPRET 10 mg by an effect on the coprimary endpoints of pain freedom and most bothersome symptom (MBS) freedom at 2 hours after a single dose, compared to placebo. Pain freedom was defined as a reduction of moderate or severe headache pain to no headache pain, and MBS freedom was defined as the absence of the self-identified MBS (i.e., photophobia, phonophobia, or nausea). The most common MBS reported before dosing was photophobia (55%), followed by nausea (28%), and phonophobia (16%).
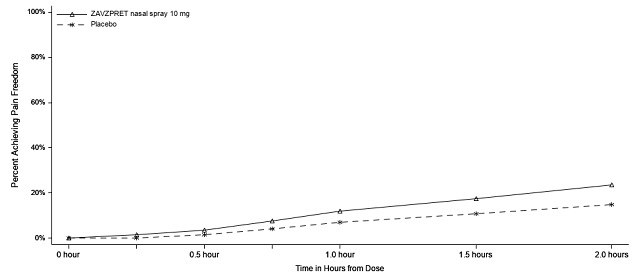
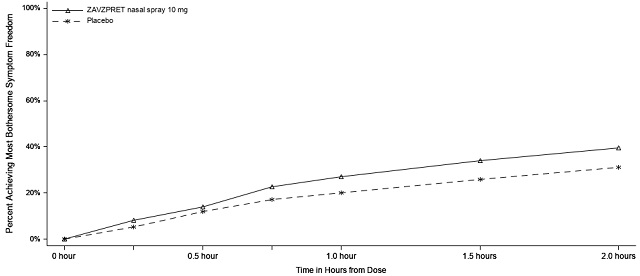
In Study 1, the percentage of patients achieving headache pain freedom and MBS freedom 2 hours after a single dose was statistically significantly greater in patients who received ZAVZPRET compared to those who received placebo (Table 2).
Table 2: Efficacy Endpoints in Study 1
ZAVZPRET 10 mg | Placebo | |
Pain Free at 2 hours | ||
n/N* | 147/623 | 96/646 |
% Responders | 23.6 | 14.9 |
Difference from placebo (%) | 8.8 | |
p-value | <0.001 | |
MBS† Free at 2 hours | ||
n/N* | 247/623 | 201/646 |
% Responders | 39.6 | 31.1 |
Difference from placebo (%) | 8.7 | |
p-value | 0.001 | |
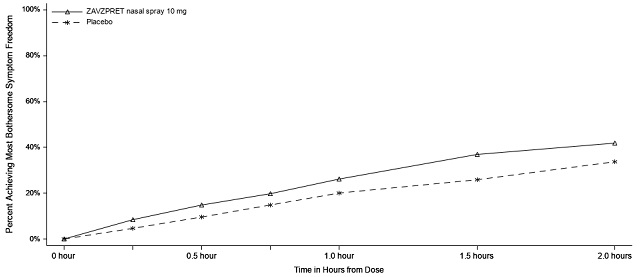
Figures 1 and 2 present the percentage of patients achieving migraine pain freedom and MBS freedom within 2 hours following treatment in Study 1.
Figure 1: Percentage of Patients Achieving Pain Freedom within 2 Hours in Study 1
Figure 2: Percentage of Patients Achieving MBS Freedom within 2 Hours in Study 1
In Study 1, statistically significant effects of ZAVZPRET compared to placebo were demonstrated for the additional efficacy endpoints of pain relief at 2 hours post-dose, return to normal function at 2 hours post-dose, sustained pain freedom from 2 to 48 hours post-dose (Table 3), and phonophobia and photophobia freedom at 2 hours post-dose. Pain relief was defined as a reduction in migraine pain from moderate or severe severity to mild or none. The measurement of the percentage of patients reporting normal function at two hours after dosing was derived from a single item questionnaire, asking patients to select one response on a 4-point scale: normal function, mild impairment, severe impairment, or required bedrest.
Table 3: Additional Efficacy Endpoints in Study 1
ZAVZPRET 10 mg | Placebo | |
Pain Relief at 2 hours | ||
n/N* | 366/623 | 321/646 |
% Responders | 58.7 | 49.7 |
Difference from placebo (%) | 9.0 | |
p-value | 0.001 | |
Percentage of Patients Reporting Normal Function at 2 hours† | ||
n/N* | 204/570 | 152/593 |
% Responders | 35.8 | 25.6 |
Difference from placebo (%) | 10.2 | |
p-value | <0.001 | |
Sustained Pain Freedom from 2 to 48 hours | ||
n/N* | 77/623 | 56/646 |
% Responders | 12.4 | 8.7 |
Difference from placebo (%) | 3.7 | |
p-value | 0.031 | |
The incidence of photophobia and phonophobia was reduced following administration of ZAVZPRET 10 mg as compared to placebo.
In Study 2 (NCT03872453), patients were randomized to receive a single dose of ZAVZPRET 10 mg (n=391) or placebo (n=401).
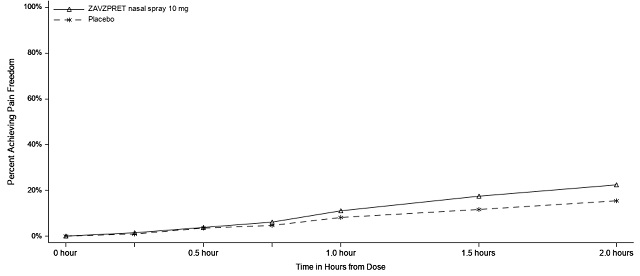
In Study 2, statistically significant efficacy was demonstrated with ZAVZPRET 10 mg by an effect on the coprimary endpoints of pain freedom and most bothersome symptom (MBS) freedom at 2 hours after a single dose, compared to placebo. Pain freedom was observed in 22.5% of patients receiving ZAVZPRET and 15.5% of patients receiving placebo (p-value = 0.011). MBS freedom was observed in 41.9% of patients receiving ZAVZPRET and 33.7% of patients receiving placebo (p-value = 0.016). The most common MBS reported before dosing was photophobia (53%), followed by nausea (31%), and phonophobia (15%).
Table 4: Efficacy Endpoints in Study 2
ZAVZPRET 10 mg | Placebo | |
Pain Free at 2 hours | ||
n/N* | 88/391 | 62/401 |
% Responders | 22.5 | 15.5 |
Difference from placebo (%) | 7.0 | |
p-value | 0.011 | |
MBS† Free at 2 hours | ||
n/N* | 164/391 | 135/401 |
% Responders | 41.9 | 33.7 |
Difference from placebo (%) | 8.3 | |
p-value | 0.016 | |
Figure 3: Percentage of Patients Achieving Pain Freedom within 2 Hours in Study 2
Figure 4: Percentage of Patients Achieving MBS Freedom within 2 Hours in Study 2
How Supplied/Storage and Handling
Medication Guide
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Hypersensitivity Reactions
Inform patients about the signs and symptoms of hypersensitivity reactions after administration of ZAVZPRET. Advise patients to contact their healthcare provider immediately if signs or symptoms of hypersensitivity reactions occur [see Warnings and Precautions (5.1)].
Drug Interactions
Advise patients to speak with their healthcare provider about any prescription or over-the-counter medications or herbal supplements that they take or plan to take. Inform patients that if they need to use an intranasal decongestant it should be administered at least 1 hour after ZAVZPRET administration [see Drug Interactions (7.3)].
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
LAB-1544-1.0
Patient Package Insert
This Patient Information has been approved by the US. Food and Drug Administration | 3/2023 |
PATIENT INFORMATION (zavegepant) nasal spray | |
What is ZAVZPRET? ZAVZPRET is a prescription medicine used in adults for the acute treatment of migraine attacks with or without aura. ZAVZPRET is not used to prevent migraine attacks. It is not known if ZAVZPRET is safe and effective in children. | |
Do not use ZAVZPRET if you are:
See the end of this leaflet for a complete list of ingredients in ZAVZPRET. | |
Before you use ZAVZPRET, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | |
How should I use ZAVZPRET?
| |
What are the possible side effects of ZAVZPRET? ZAVZPRET may cause serious side effects including:
The most common side effects of ZAVZPRET are:
These are not the only possible side effects of ZAVZPRET. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1 800-FDA-1088. | |
How should I store ZAVZPRET?
Keep ZAVZPRET and all medicines out of the reach of children. | |
General information about the safe and effective use of ZAVZPRET: Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ZAVZPRET for a condition for which it was not prescribed. Do not give ZAVZPRET to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZAVZPRET that is written for health professionals. | |
What are the ingredients in ZAVZPRET? Active ingredients in ZAVZPRET: zavegepant Inactive ingredients in ZAVZPRET: dextrose, hydrochloric acid, sodium hydroxide, and succinic acid in water for injection.  For more information, go to www.zavzpret.com or call 1-800-438-1985. LAB-1545-1.0 | |
Instructions For Use
Instructions for Use
INSTRUCTIONS FOR USE ZAVZPRET [zav-spret] (zavegepant) nasal spray For Nasal Use Only | ||
This Instructions for Use contains information on how to give a single dose (10 mg) with ZAVZPRET nasal spray One (1) device delivers a single dose of ZAVZPRET. The device provides 1 spray that should be delivered into 1 nostril. | ||
ZAVZPRET Device Parts  | ||
Important Information You Need to Know Before Dosing with ZAVZPRET
Storage Information
Preparing to Dose with ZAVZPRET Nasal Spray | ||
1 | Gently Blow Your Nose | |
1.1 | Before using ZAVZPRET, blow your nose to clear your nostrils (see Figure B). This can be done while sitting or standing. | |
 | ||
2 | Remove 1 Blister from the Carton | |
2.1 | Remove 1 blister containing a device from the carton (see Figure C). | |
 | ||
3 | Check Expiration Date on the Blister | |
3.1 | Check the expiration date on the blister containing a device (see Figure D). | |
Do not use if the expiration date has passed. Throw away the expired device in the trash if the expiration date has passed. | ||
 | ||
4 | Remove Device from Blister | |
4.1 | Peel open the blister seal from the corner and remove it completely (see Figure E). | |
4.2 | Carefully remove the device from the plastic tray. | |
 | ||
Dosing with ZAVZPRET Nasal Spray | ||
5 | Check Expiration Date on the Device | |
5.1 | Check the expiration date on the device (see Figure F). Do not use if the expiration date has passed. Throw away the expired device in the trash if the expiration date has passed. | |
 | ||
6 | Grip the Device | |
6.1 | Hold the device upright with your thumb on the bottom of the plunger and two fingers on either side of the nozzle (see Figure G). Do not press the plunger yet. If you press the plunger now, the medicine will spray and be wasted. | |
 | ||
Dosing with ZAVZPRET Nasal Spray (Continued) | ||
7 | Close 1 Nostril | |
7.1 | With your free hand, gently press 1 nostril to close it (see Figure H). | |
7.2 | Continue breathing normally through your mouth. | |
 | ||
8 | Insert Spray Nozzle Into Open Nostril | |
8.1 | Insert the nozzle into the open nostril as much as you comfortably can (see Figure I). | |
 | ||
9 | Deliver the Dose by Spraying Into Nostril and Breathing In | |
9.1 | Keep your head level and upright and close your mouth. Do not tilt your head. Do not lay down while delivering the dose. | |
9.2 | Slowly breathe in through your nose as you firmly press the plunger up with your thumb to release the spray (see Figure J). Important: Hold the nozzle firmly in your nose as you deliver the dose. Do not let the nozzle come out when pressing the plunger. Spray only 1 time into 1 nostril. | |
 | ||
10 | Remove the Device and Keep Your Head Level for 20 Seconds | |
10.1 | Remove the device from your nostril and your finger from your other nostril. | |
10.2 | While keeping your head level and upright, gently breathe in through your nose and out through your mouth for 10 to 20 seconds (see Figure K). | |
If you feel a drip from your nose, gently sniff so you do not lose any of your dose. | ||
 | ||
Throwing away (Disposing of) Device | ||
11 | Throw Away Used Device | |
11.1 | Throw away the used device into trash (see Figure L) | |
 | ||
You have received your full dose, if you have:
Additional Information For more information on ZAVZPRET (zavegepant), visit www.zavzpret.com or call 1-800-438-1985. This Instructions for Use has been approved by the U.S. Food and Drug Administration Approved: 3/2023  LAB-1546-1.0 | ||
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information. 9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.