Rocuronium bromide (NDC 0409-9558-05 and 0409-9558-10)
(rocuronium bromide)
Find Rocuronium bromide (NDC 0409-9558-05 and 0409-9558-10) medical information:
Find Rocuronium bromide (NDC 0409-9558-05 and 0409-9558-10) medical information:
Rocuronium bromide (NDC 0409-9558-05 and 0409-9558-10) Quick Finder
17 PATIENT COUNSELING INFORMATION
17 PATIENT COUNSELING INFORMATION
Obtain information about your patient's medical history, current medications, any history of hypersensitivity to rocuronium bromide or other neuromuscular blocking agents. If applicable, inform your patients that certain medical conditions and medications might influence how rocuronium bromide works.
In addition, inform your patient that severe anaphylactic reactions to neuromuscular blocking agents, including rocuronium bromide, have been reported. Since allergic cross-reactivity has been reported in this class, request information from your patients about previous anaphylactic reactions to other neuromuscular blocking agents.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA 
LAB-1239-4.0
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Obtain information about your patient's medical history, current medications, any history of hypersensitivity to rocuronium bromide or other neuromuscular blocking agents. If applicable, inform your patients that certain medical conditions and medications might influence how rocuronium bromide works.
In addition, inform your patient that severe anaphylactic reactions to neuromuscular blocking agents, including rocuronium bromide, have been reported. Since allergic cross-reactivity has been reported in this class, request information from your patients about previous anaphylactic reactions to other neuromuscular blocking agents.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA 
LAB-1239-4.0
Highlights
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ROCURONIUM BROMIDE INJECTION safely and effectively. See full prescribing information for ROCURONIUM BROMIDE INJECTION. ROCURONIUM BROMIDE injection solution for intravenous use Initial U.S. Approval: 1994 RECENT MAJOR CHANGESINDICATIONS AND USAGERocuronium bromide injection is a nondepolarizing neuromuscular blocking agent indicated as an adjunct to general anesthesia to facilitate both rapid sequence and routine tracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation. (1) DOSAGE AND ADMINISTRATIONTo be administered only by experienced clinicians or adequately trained individuals supervised by an experienced clinician familiar with the use, actions, characteristics, and complications of neuromuscular blocking agents. (2.1)
DOSAGE FORMS AND STRENGTHSCONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (2%) are transient hypotension and hypertension. (6) To report SUSPECTED ADVERSE REACTIONS, contact Hospira, Inc. at 1-800-441-4100 or electronically at ProductComplaintsPP@hospira.com, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2019 |
Indications and Usage
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing and Administration Information
Rocuronium bromide injection is for intravenous use only. This drug should only be administered by experienced clinicians or trained individuals supervised by an experienced clinician familiar with the use, actions, characteristics, and complications of neuromuscular blocking agents. Doses of rocuronium bromide injection should be individualized and a peripheral nerve stimulator should be used to monitor drug effect, need for additional doses, adequacy of spontaneous recovery or antagonism, and to decrease the complications of overdosage if additional doses are administered.
The dosage information which follows is derived from studies based upon units of drug per unit of body weight. It is intended to serve as an initial guide to clinicians familiar with other neuromuscular blocking agents to acquire experience with rocuronium bromide injection.
In patients in whom potentiation of, or resistance to, neuromuscular block is anticipated, a dose adjustment should be considered [see Dosage and Administration (2.6), Warnings and Precautions (5.10, 5.13), Drug Interactions (7.2, 7.3, 7.4, 7.5, 7.6, 7.8, 7.10), and Use in Specific Populations (8.6)].
Risk of Medication Errors
Accidental administration of neuromuscular blocking agents may be fatal. Store rocuronium bromide with the cap and ferrule intact and in a manner that minimizes the possibility of selecting the wrong product [see Warnings and Precautions (5.3)].
2.2 Dose for Tracheal Intubation
The recommended initial dose of rocuronium bromide injection, regardless of anesthetic technique, is 0.6 mg/kg. Neuromuscular block sufficient for intubation (80% block or greater) is attained in a median (range) time of 1 (0.4 to 6) minute(s) and most patients have intubation completed within 2 minutes. Maximum blockade is achieved in most patients in less than 3 minutes. This dose may be expected to provide 31 (15 to 85) minutes of clinical relaxation under opioid/nitrous oxide/oxygen anesthesia. Under halothane, isoflurane, and enflurane anesthesia, some extension of the period of clinical relaxation should be expected [see Drug Interactions (7.3)].
A lower dose of rocuronium bromide injection (0.45 mg/kg) may be used. Neuromuscular block sufficient for intubation (80% block or greater) is attained in a median (range) time of 1.3 (0.8 to 6.2) minute(s), and most patients have intubation completed within 2 minutes. Maximum blockade is achieved in most patients in less than 4 minutes. This dose may be expected to provide 22 (12 to 31) minutes of clinical relaxation under opioid/nitrous oxide/oxygen anesthesia. Patients receiving this low dose of 0.45 mg/kg who achieve less than 90% block (about 16% of these patients) may have a more rapid time to 25% recovery, 12 to 15 minutes.
A large bolus dose of 0.9 or 1.2 mg/kg can be administered under opioid/nitrous oxide/oxygen anesthesia without adverse effects to the cardiovascular system [see Clinical Pharmacology (12.2)].
2.3 Rapid Sequence Intubation
In appropriately premedicated and adequately anesthetized patients, rocuronium bromide injection 0.6 to 1.2 mg/kg will provide excellent or good intubating conditions in most patients in less than 2 minutes [see Clinical Studies (14.1)].
2.4 Maintenance Dosing
Maintenance doses of 0.1, 0.15, and 0.2 mg/kg rocuronium bromide injection, administered at 25% recovery of control T1 (defined as 3 twitches of train-of-four), provide a median (range) of 12 (2 to 31), 17 (6 to 50) and 24 (7 to 69) minutes of clinical duration under opioid/nitrous oxide/oxygen anesthesia [see Clinical Pharmacology (12.2)]. In all cases, dosing should be guided based on the clinical duration following initial dose or prior maintenance dose and not administered until recovery of neuromuscular function is evident. A clinically insignificant cumulation of effect with repetitive maintenance dosing has been observed [see Clinical Pharmacology (12.2)].
2.5 Use by Continuous Infusion
Infusion at an initial rate of 10 to 12 mcg/kg/min of rocuronium bromide injection should be initiated only after early evidence of spontaneous recovery from an intubating dose. Due to rapid redistribution [see Clinical Pharmacology (12.3)] and the associated rapid spontaneous recovery, initiation of the infusion after substantial return of neuromuscular function (more than 10% of control T1), may necessitate additional bolus doses to maintain adequate block for surgery.
Upon reaching the desired level of neuromuscular block, the infusion of rocuronium bromide injection must be individualized for each patient. The rate of administration should be adjusted according to the patient's twitch response as monitored with the use of a peripheral nerve stimulator. In clinical trials, infusion rates have ranged from 4 to 16 mcg/kg/min.
Inhalation anesthetics, particularly enflurane and isoflurane, may enhance the neuromuscular blocking action of nondepolarizing muscle relaxants. In the presence of steady-state concentrations of enflurane or isoflurane, it may be necessary to reduce the rate of infusion by 30% to 50%, at 45 to 60 minutes after the intubating dose.
Spontaneous recovery and reversal of neuromuscular blockade following discontinuation of rocuronium bromide infusion may be expected to proceed at rates comparable to that following comparable total doses administered by repetitive bolus injections [see Clinical Pharmacology (12.2)].
Infusion solutions of rocuronium bromide injection can be prepared by mixing rocuronium bromide injection with an appropriate infusion solution such as 5% glucose in water or lactated Ringers [see Dosage and Administration (2.7)]. These infusion solutions should be used within 24 hours of mixing. Unused portions of infusion solutions should be discarded.
Infusion rates of rocuronium bromide injection can be individualized for each patient using the following tables for 3 different concentrations of rocuronium bromide injection solution as guidelines:
| * 50 mg rocuronium bromide injection in 100 mL solution | |||||||||||
Patient Weight | Drug Delivery Rate (mcg/kg/min) | ||||||||||
(kg) | (lbs) | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 12 | 14 | 16 |
Infusion Delivery Rate (mL/hr) | |||||||||||
10 | 22 | 4.8 | 6 | 7.2 | 8.4 | 9.6 | 10.8 | 12 | 14.4 | 16.8 | 19.2 |
15 | 33 | 7.2 | 9 | 10.8 | 12.6 | 14.4 | 16.2 | 18 | 21.6 | 25.2 | 28.8 |
20 | 44 | 9.6 | 12 | 14.4 | 16.8 | 19.2 | 21.6 | 24 | 28.8 | 33.6 | 38.4 |
25 | 55 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 36 | 42 | 48 |
35 | 77 | 16.8 | 21 | 25.2 | 29.4 | 33.6 | 37.8 | 42 | 50.4 | 58.8 | 67.2 |
50 | 110 | 24 | 30 | 36 | 42 | 48 | 54 | 60 | 72 | 84 | 96 |
60 | 132 | 28.8 | 36 | 43.2 | 50.4 | 57.6 | 64.8 | 72 | 86.4 | 100.8 | 115.2 |
70 | 154 | 33.6 | 42 | 50.4 | 58.8 | 67.2 | 75.6 | 84 | 100.8 | 117.6 | 134.4 |
80 | 176 | 38.4 | 48 | 57.6 | 67.2 | 76.8 | 86.4 | 96 | 115.2 | 134.4 | 153.6 |
90 | 198 | 43.2 | 54 | 64.8 | 75.6 | 86.4 | 97.2 | 108 | 129.6 | 151.2 | 172.8 |
100 | 220 | 48 | 60 | 72 | 84 | 96 | 108 | 120 | 144 | 168 | 192 |
| ** 100 mg rocuronium bromide injection in 100 mL solution | |||||||||||||||||||||||
Patient Weight | Drug Delivery Rate (mcg/kg/min) | ||||||||||||||||||||||
(kg) | (lbs) | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 12 | 14 | 16 | ||||||||||||
Infusion Delivery Rate (mL/hr) | |||||||||||||||||||||||
10 | 22 | 2.4 | 3 | 3.6 | 4.2 | 4.8 | 5.4 | 6 | 7.2 | 8.4 | 9.6 | ||||||||||||
15 | 33 | 3.6 | 4.5 | 5.4 | 6.3 | 7.2 | 8.1 | 9 | 10.8 | 12.6 | 14.4 | ||||||||||||
20 | 44 | 4.8 | 6 | 7.2 | 8.4 | 9.6 | 10.8 | 12 | 14.4 | 16.8 | 19.2 | ||||||||||||
25 | 55 | 6 | 7.5 | 9 | 10.5 | 12 | 13.5 | 15 | 18 | 21 | 24 | ||||||||||||
35 | 77 | 8.4 | 10.5 | 12.6 | 14.7 | 16.8 | 18.9 | 21 | 25.2 | 29.4 | 33.6 | ||||||||||||
50 | 110 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 36 | 42 | 48 | ||||||||||||
60 | 132 | 14.4 | 18 | 21.6 | 25.2 | 28.8 | 32.4 | 36 | 43.2 | 50.4 | 57.6 | ||||||||||||
70 | 154 | 16.8 | 21 | 25.2 | 29.4 | 33.6 | 37.8 | 42 | 50.4 | 58.8 | 67.2 | ||||||||||||
80 | 176 | 19.2 | 24 | 28.8 | 33.6 | 38.4 | 43.2 | 48 | 57.6 | 67.2 | 76.8 | ||||||||||||
90 | 198 | 21.6 | 27 | 32.4 | 37.8 | 43.2 | 48.6 | 54 | 64.8 | 75.6 | 86.4 | ||||||||||||
100 | 220 | 24 | 30 | 36 | 42 | 48 | 54 | 60 | 72 | 84 | 96 | ||||||||||||
| *** 500 mg rocuronium bromide injection in 100 mL solution | |||||||||||||||||||||||||||||||||||
Patient Weight | Drug Delivery Rate (mcg/kg/min) | ||||||||||||||||||||||||||||||||||
(kg) | (lbs) | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 12 | 14 | 16 | ||||||||||||||||||||||||
Infusion Delivery Rate (mL/hr) | |||||||||||||||||||||||||||||||||||
10 | 22 | 0.5 | 0.6 | 0.7 | 0.8 | 1 | 1.1 | 1.2 | 1.4 | 1.7 | 1.9 | ||||||||||||||||||||||||
15 | 33 | 0.7 | 0.9 | 1.1 | 1.3 | 1.4 | 1.6 | 1.8 | 2.2 | 2.5 | 2.9 | ||||||||||||||||||||||||
20 | 44 | 1 | 1.2 | 1.4 | 1.7 | 1.9 | 2.2 | 2.4 | 2.9 | 3.4 | 3.8 | ||||||||||||||||||||||||
25 | 55 | 1.2 | 1.5 | 1.8 | 2.1 | 2.4 | 2.7 | 3 | 3.6 | 4.2 | 4.8 | ||||||||||||||||||||||||
35 | 77 | 1.7 | 2.1 | 2.5 | 2.9 | 3.4 | 3.8 | 4.2 | 5 | 5.9 | 6.7 | ||||||||||||||||||||||||
50 | 110 | 2.4 | 3 | 3.6 | 4.2 | 4.8 | 5.4 | 6 | 7.2 | 8.4 | 9.6 | ||||||||||||||||||||||||
60 | 132 | 2.9 | 3.6 | 4.3 | 5 | 5.8 | 6.5 | 7.2 | 8.6 | 10.1 | 11.5 | ||||||||||||||||||||||||
70 | 154 | 3.4 | 4.2 | 5 | 5.9 | 6.7 | 7.6 | 8.4 | 10.1 | 11.8 | 13.4 | ||||||||||||||||||||||||
80 | 176 | 3.8 | 4.8 | 5.8 | 6.7 | 7.7 | 8.6 | 9.6 | 11.5 | 13.4 | 15.4 | ||||||||||||||||||||||||
90 | 198 | 4.3 | 5.4 | 6.5 | 7.6 | 8.6 | 9.7 | 10.8 | 13 | 15.1 | 17.3 | ||||||||||||||||||||||||
100 | 220 | 4.8 | 6 | 7.2 | 8.4 | 9.6 | 10.8 | 12 | 14.4 | 16.8 | 19.2 | ||||||||||||||||||||||||
2.6 Dosage in Specific Populations
Pediatric Patients
The recommended initial intubation dose of rocuronium bromide injection is 0.6 mg/kg; however, a lower dose of 0.45 mg/kg may be used depending on anesthetic technique and the age of the patient.
For sevoflurane (induction) rocuronium bromide doses of 0.45 mg/kg and 0.6 mg/kg in general produce excellent to good intubating conditions within 75 seconds. When halothane is used, a 0.6 mg/kg dose of rocuronium bromide injection resulted in excellent to good intubating conditions within 60 seconds.
The time to maximum block for an intubating dose was shortest in infants (28 days up to 3 months) and longest in neonates (birth to less than 28 days). The duration of clinical relaxation following an intubating dose is shortest in children (greater than 2 years up to 11 years) and longest in infants.
When sevoflurane is used for induction and isoflurane/nitrous oxide for maintenance of general anesthesia, maintenance dosing of rocuronium bromide injection can be administered as bolus doses of 0.15 mg/kg at reappearance of T3 in all pediatric age groups. Maintenance dosing can also be administered at the reappearance of T2 at a rate of 7 to 10 mcg/kg/min, with the lowest dose requirement for neonates (birth to less than 28 days) and the highest dose requirement for children (greater than 2 years up to 11 years).
When halothane is used for general anesthesia, patients ranging from 3 months old through adolescence can be administered rocuronium bromide injection maintenance doses of 0.075 to 0.125 mg/kg upon return of T1 to 0.25% to provide clinical relaxation for 7 to 10 minutes. Alternatively, a continuous infusion of rocuronium bromide injection initiated at a rate of 12 mcg/kg/min upon return of T1 to 10% (one twitch present in train-of-four) may also be used to maintain neuromuscular blockade in pediatric patients.
Additional information for administration to pediatric patients of all age groups is presented elsewhere in the label [see Clinical Pharmacology (12.2)].
The infusion of rocuronium bromide injection must be individualized for each patient. The rate of administration should be adjusted according to the patient's twitch response as monitored with the use of a peripheral nerve stimulator. Spontaneous recovery and reversal of neuromuscular blockade following discontinuation of rocuronium bromide infusion may be expected to proceed at rates comparable to that following similar total exposure to single bolus doses [see Clinical Pharmacology (12.2)].
Rocuronium bromide injection is not recommended for rapid sequence intubation in pediatric patients.
Geriatric Patients
Geriatric patients (65 years or older) exhibited a slightly prolonged median (range) clinical duration of 46 (22 to 73), 62 (49 to 75), and 94 (64 to 138) minutes under opioid/nitrous oxide/oxygen anesthesia following doses of 0.6, 0.9, and 1.2 mg/kg, respectively. No differences in duration of neuromuscular blockade following maintenance doses of rocuronium bromide injection were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out [see Clinical Pharmacology (12.2) and Clinical Studies (14.2)]. [See also Warnings and Precautions (5.5)].
Patients with Renal or Hepatic Impairment
No differences from patients with normal hepatic and kidney function were observed for onset time at a dose of 0.6 mg/kg rocuronium bromide injection. When compared to patients with normal renal and hepatic function, the mean clinical duration is similar in patients with end-stage renal disease undergoing renal transplant, and is about 1.5 times longer in patients with hepatic disease. Patients with renal failure may have a greater variation in duration of effect [see Use in Specific Populations (8.6, 8.7) and Clinical Pharmacology (12.3)].
Obese Patients
In obese patients, the initial dose of rocuronium bromide injection 0.6 mg/kg should be based upon the patient's actual body weight [see Clinical Studies (14.1)].
An analysis across all US controlled clinical studies indicates that the pharmacodynamics of rocuronium bromide injection are not different between obese and nonobese patients when dosed based upon their actual body weight.
Patients with Reduced Plasma Cholinesterase Activity
Rocuronium metabolism does not depend on plasma cholinesterase so dosing adjustments are not needed in patients with reduced plasma cholinesterase activity.
Patients with Prolonged Circulation Time
Because higher doses of rocuronium bromide injection produce a longer duration of action, the initial dosage should usually not be increased in these patients to reduce onset time; instead, in these situations, when feasible, more time should be allowed for the drug to achieve onset of effect [see Warnings and Precautions (5.8)].
Patients with Drugs or Conditions Causing Potentiation of Neuromuscular Block
The neuromuscular blocking action of rocuronium bromide injection is potentiated by isoflurane and enflurane anesthesia. Potentiation is minimal when administration of the recommended dose of rocuronium bromide injection occurs prior to the administration of these potent inhalation agents. The median clinical duration of a dose of 0.57 to 0.85 mg/kg was 34, 38, and 42 minutes under opioid/nitrous oxide/oxygen, enflurane and isoflurane maintenance anesthesia, respectively. During 1 to 2 hours of infusion, the infusion rate of rocuronium bromide injection required to maintain about 95% block was decreased by as much as 40% under enflurane and isoflurane anesthesia [see Drug Interactions (7.3)].
2.7 Preparation for Administration of Rocuronium Bromide Injection
Diluent Compatibility
Rocuronium bromide injection is compatible in solution with:
0.9% NaCl solution sterile water for injection
5% glucose in water lactated Ringers
5% glucose in saline
Rocuronium bromide injection is compatible in the above solutions at concentrations up to 5 mg/mL for 24 hours at room temperature in plastic bags, glass bottles, and plastic syringe pumps.
Drug Admixture Incompatibility
Rocuronium bromide injection is physically incompatible when mixed with the following drugs:
amphotericin hydrocortisone sodium succinate
amoxicillin insulin
azathioprine intralipid
cefazolin ketorolac
cloxacillin lorazepam
dexamethasone methohexital
diazepam methylprednisolone
erythromycin thiopental
famotidine trimethoprim
furosemide vancomycin
If rocuronium bromide injection is administered via the same infusion line that is also used for other drugs, it is important that this infusion line is adequately flushed between administration of rocuronium bromide injection and drugs for which incompatibility with rocuronium bromide injection has been demonstrated or for which compatibility with rocuronium bromide injection has not been established.
Infusion solutions should be used within 24 hours of mixing. Unused portions of infusion solutions should be discarded.
Rocuronium bromide injection should not be mixed with alkaline solutions [see Warnings and Precautions (5.11)].
Visual Inspection
Parenteral drug products should be inspected visually for particulate matter and clarity prior to administration whenever solution and container permit. Do not use solution if particulate matter is present.
Dosage Forms and Strengths
Contraindications
4 CONTRAINDICATIONS
Rocuronium bromide is contraindicated in patients known to have hypersensitivity (e.g., anaphylaxis) to rocuronium bromide or other neuromuscular blocking agents [see Warnings and Precautions (5.2)].
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Appropriate Administration and Monitoring
Rocuronium bromide should be administered in carefully adjusted dosages by or under the supervision of experienced clinicians who are familiar with the drug's actions and the possible complications of its use. The drug should not be administered unless facilities for intubation, mechanical ventilation, oxygen therapy, and an antagonist are immediately available. It is recommended that clinicians administering neuromuscular blocking agents such as rocuronium bromide employ a peripheral nerve stimulator to monitor drug effect, need for additional doses, adequacy of spontaneous recovery or antagonism, and to decrease the complications of overdosage if additional doses are administered.
5.2 Anaphylaxis
Severe anaphylactic reactions to neuromuscular blocking agents, including rocuronium bromide, have been reported. These reactions have, in some cases (including cases with rocuronium bromide), been life threatening and fatal. Due to the potential severity of these reactions, the necessary precautions, such as the immediate availability of appropriate emergency treatment, should be taken. Precautions should also be taken in those patients who have had previous anaphylactic reactions to other neuromuscular blocking agents, since cross-reactivity between neuromuscular blocking agents, both depolarizing and nondepolarizing, has been reported.
5.3 Risk of Death due to Medication Errors
Administration of rocuronium bromide results in paralysis, which may lead to respiratory arrest and death, a progression that may be more likely to occur in a patient for whom it is not intended. Confirm proper selection of intended product and avoid confusion with other injectable solutions that are present in critical care and other clinical settings. If another healthcare provider is administering the product, ensure that the intended dose is clearly labeled and communicated.
5.4 Need for Adequate Anesthesia
Rocuronium bromide has no known effect on consciousness, pain threshold, or cerebration. Therefore, its administration must be accompanied by adequate anesthesia or sedation.
5.5 Residual Paralysis
In order to prevent complications resulting from residual paralysis, it is recommended to extubate only after the patient has recovered sufficiently from neuromuscular block. Geriatric patients (65 years or older) may be at increased risk for residual neuromuscular block. Other factors which could cause residual paralysis after extubation in the post-operative phase (such as drug interactions or patient condition) should also be considered. If not used as part of standard clinical practice the use of a reversal agent should be considered, especially in those cases where residual paralysis is more likely to occur.
5.6 Long-Term Use in an Intensive Care Unit
Rocuronium bromide has not been studied for long-term use in the intensive care unit (ICU). As with other nondepolarizing neuromuscular blocking drugs, apparent tolerance to rocuronium bromide may develop during chronic administration in the ICU. While the mechanism for development of this resistance is not known, receptor up-regulation may be a contributing factor. It is strongly recommended that neuromuscular transmission be monitored continuously during administration and recovery with the help of a nerve stimulator. Additional doses of rocuronium bromide or any other neuromuscular blocking agent should not be given until there is a definite response (one twitch of the train-of-four) to nerve stimulation. Prolonged paralysis and/or skeletal muscle weakness may be noted during initial attempts to wean from the ventilator patients who have chronically received neuromuscular blocking drugs in the ICU.
Myopathy after long-term administration of other nondepolarizing neuromuscular blocking agents in the ICU alone or in combination with corticosteroid therapy has been reported. Therefore, for patients receiving both neuromuscular blocking agents and corticosteroids, the period of use of the neuromuscular blocking agent should be limited as much as possible and only used in the setting where in the opinion of the prescribing physician, the specific advantages of the drug outweigh the risk.
5.7 Malignant Hyperthermia (MH)
Rocuronium bromide has not been studied in MH-susceptible patients. Because rocuronium bromide is always used with other agents, and the occurrence of malignant hyperthermia during anesthesia is possible even in the absence of known triggering agents, clinicians should be familiar with early signs, confirmatory diagnosis, and treatment of malignant hyperthermia prior to the start of any anesthetic [see Adverse Reactions (6.2)].
In an animal study in MH-susceptible swine, the administration of rocuronium bromide injection did not appear to trigger malignant hyperthermia.
5.8 Prolonged Circulation Time
Conditions associated with an increased circulatory delayed time, e.g., cardiovascular disease or advanced age, may be associated with a delay in onset time [see Dosage and Administration (2.6)].
5.9 QT Interval Prolongation
The overall analysis of ECG data in pediatric patients indicates that the concomitant use of rocuronium bromide with general anesthetic agents can prolong the QTc interval [see Clinical Studies (14.3)].
5.10 Conditions/Drugs Causing Potentiation of, or Resistance to, Neuromuscular Block
Potentiation
Nondepolarizing neuromuscular blocking agents have been found to exhibit profound neuromuscular blocking effects in cachectic or debilitated patients, patients with neuromuscular diseases, and patients with carcinomatosis.
Certain inhalation anesthetics, particularly enflurane and isoflurane, antibiotics, magnesium salts, lithium, local anesthetics, procainamide, and quinidine have been shown to increase the duration of neuromuscular block and decrease infusion requirements of neuromuscular blocking agents [see Drug Interactions (7.3)].
In these or other patients in whom potentiation of neuromuscular block or difficulty with reversal may be anticipated, a decrease from the recommended initial dose of rocuronium bromide should be considered [see Dosage and Administration (2.6)].
Resistance
Resistance to nondepolarizing agents, consistent with up-regulation of skeletal muscle acetylcholine receptors, is associated with burns, disuse atrophy, denervation, and direct muscle trauma. Receptor up-regulation may also contribute to the resistance to nondepolarizing muscle relaxants which sometimes develops in patients with cerebral palsy, patients chronically receiving anticonvulsant agents such as carbamazepine or phenytoin, or with chronic exposure to nondepolarizing agents. When rocuronium bromide is administered to these patients, shorter durations of neuromuscular block may occur, and infusion rates may be higher due to the development of resistance to nondepolarizing muscle relaxants.
Potentiation or Resistance
Severe acid-base and/or electrolyte abnormalities may potentiate or cause resistance to the neuromuscular blocking action of rocuronium bromide. No data are available in such patients and no dosing recommendations can be made.
Rocuronium bromide-induced neuromuscular blockade was modified by alkalosis and acidosis in experimental pigs. Both respiratory and metabolic acidosis prolonged the recovery time. The potency of rocuronium bromide was significantly enhanced in metabolic acidosis and alkalosis, but was reduced in respiratory alkalosis. In addition, experience with other drugs has suggested that acute (e.g., diarrhea) or chronic (e.g., adrenocortical insufficiency) electrolyte imbalance may alter neuromuscular blockade. Since electrolyte imbalance and acid-base imbalance are usually mixed, either enhancement or inhibition may occur.
5.11 Incompatibility with Alkaline Solutions
Rocuronium bromide, which has an acid pH, should not be mixed with alkaline solutions (e.g., barbiturate solutions) in the same syringe or administered simultaneously during intravenous infusion through the same needle.
5.12 Increase in Pulmonary Vascular Resistance
Rocuronium bromide may be associated with increased pulmonary vascular resistance, so caution is appropriate in patients with pulmonary hypertension or valvular heart disease [see Clinical Studies (14.1)].
5.13 Use In Patients with Myasthenia
In patients with myasthenia gravis or myasthenic (Eaton-Lambert) syndrome, small doses of nondepolarizing neuromuscular blocking agents may have profound effects. In such patients, a peripheral nerve stimulator and use of a small test dose may be of value in monitoring the response to administration of muscle relaxants.
Adverse Reactions
6 ADVERSE REACTIONS
In clinical trials, the most common adverse reactions (2%) are transient hypotension and hypertension.
The following adverse reactions are described, or described in greater detail, in other sections:
- •
- Anaphylaxis [see Warnings and Precautions (5.2)]
- •
- Residual paralysis [see Warnings and Precautions (5.5)]
- •
- Myopathy [see Warnings and Precautions (5.6)]
- •
- Increased pulmonary vascular resistance [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical studies in the US (n=1137) and Europe (n=1394) totaled 2531 patients. The patients exposed in the US clinical studies provide the basis for calculation of adverse reaction rates. The following adverse reactions were reported in patients administered rocuronium bromide (all events judged by investigators during the clinical trials to have a possible causal relationship):
Adverse reactions in greater than 1% of patients: None
Adverse reactions in less than 1% of patients (probably related or relationship unknown):
Cardiovascular: arrhythmia, abnormal electrocardiogram, tachycardia
Digestive: nausea, vomiting
Respiratory: asthma (bronchospasm, wheezing, or rhonchi), hiccup
Skin and Appendages: rash, injection site edema, pruritus
In the European studies, the most commonly reported reactions were transient hypotension (2%) and hypertension (2%); these are in greater frequency than the US studies (0.1% and 0.1%). Changes in heart rate and blood pressure were defined differently from in the US studies in which changes in cardiovascular parameters were not considered as adverse events unless judged by the investigator as unexpected, clinically significant, or thought to be histamine related.
In a clinical study in patients with clinically significant cardiovascular disease undergoing coronary artery bypass graft, hypertension and tachycardia were reported in some patients, but these occurrences were less frequent in patients receiving beta or calcium channel-blocking drugs. In some patients, rocuronium bromide was associated with transient increases (30% or greater) in pulmonary vascular resistance. In another clinical study of patients undergoing abdominal aortic surgery, transient increases (30% or greater) in pulmonary vascular resistance were observed in about 24% of patients receiving rocuronium bromide 0.6 or 0.9 mg/kg.
In pediatric patient studies worldwide (n=704), tachycardia occurred at an incidence of 5.3% (n=37), and it was judged by the investigator as related in 10 cases (1.4%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of rocuronium bromide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: In clinical practice, there have been reports of severe allergic reactions (anaphylactic and anaphylactoid reactions and shock) with rocuronium bromide, including some that have been life-threatening and fatal [see Warnings and Precautions (5.2)].
General disorders and administration site conditions: There have been reports of malignant hyperthermia with the use of rocuronium bromide [see Warnings and Precautions (5.7)].
Drug Interactions
7 DRUG INTERACTIONS
7.1 Antibiotics
Drugs which may enhance the neuromuscular blocking action of nondepolarizing agents such as rocuronium bromide include certain antibiotics (e.g., aminoglycosides; vancomycin; tetracyclines; bacitracin; polymyxins; colistin; and sodium colistimethate). If these antibiotics are used in conjunction with rocuronium bromide, prolongation of neuromuscular block may occur.
7.2 Anticonvulsants
In 2 of 4 patients receiving chronic anticonvulsant therapy, apparent resistance to the effects of rocuronium bromide was observed in the form of diminished magnitude of neuromuscular block, or shortened clinical duration. As with other nondepolarizing neuromuscular blocking drugs, if rocuronium bromide is administered to patients chronically receiving anticonvulsant agents such as carbamazepine or phenytoin, shorter durations of neuromuscular block may occur and infusion rates may be higher due to the development of resistance to nondepolarizing muscle relaxants. While the mechanism for development of this resistance is not known, receptor up-regulation may be a contributing factor [see Warnings and Precautions (5.10)].
7.3 Inhalation Anesthetics
Use of inhalation anesthetics has been shown to enhance the activity of other neuromuscular blocking agents (enflurane > isoflurane > halothane).
Isoflurane and enflurane may also prolong the duration of action of initial and maintenance doses of rocuronium bromide and decrease the average infusion requirement of rocuronium bromide by 40% compared to opioid/nitrous oxide/oxygen anesthesia. No definite interaction between rocuronium bromide and halothane has been demonstrated. In one study, use of enflurane in 10 patients resulted in a 20% increase in mean clinical duration of the initial intubating dose, and a 37% increase in the duration of subsequent maintenance doses, when compared in the same study to 10 patients under opioid/nitrous oxide/oxygen anesthesia. The clinical duration of initial doses of rocuronium bromide of 0.57 to 0.85 mg/kg under enflurane or isoflurane anesthesia, as used clinically, was increased by 11% and 23%, respectively. The duration of maintenance doses was affected to a greater extent, increasing by 30% to 50% under either enflurane or isoflurane anesthesia.
Potentiation by these agents is also observed with respect to the infusion rates of rocuronium bromide required to maintain approximately 95% neuromuscular block. Under isoflurane and enflurane anesthesia, the infusion rates are decreased by approximately 40% compared to opioid/nitrous oxide/oxygen anesthesia. The median spontaneous recovery time (from 25% to 75% of control T1) is not affected by halothane, but is prolonged by enflurane (15% longer) and isoflurane (62% longer). Reversal-induced recovery of rocuronium bromide neuromuscular block is minimally affected by anesthetic technique [see Dosage and Administration (2.6) and Warnings and Precautions (5.10)].
7.4 Lithium Carbonate
Lithium has been shown to increase the duration of neuromuscular block and decrease infusion requirements of neuromuscular blocking agents [see Warnings and Precautions (5.10)].
7.5 Local Anesthetics
Local anesthetics have been shown to increase the duration of neuromuscular block and decrease infusion requirements of neuromuscular blocking agents [see Warnings and Precautions (5.10)].
7.6 Magnesium
Magnesium salts administered for the management of toxemia of pregnancy may enhance neuromuscular blockade [see Warnings and Precautions (5.10)].
7.7 Nondepolarizing Muscle Relaxants
There are no controlled studies documenting the use of rocuronium bromide before or after other nondepolarizing muscle relaxants. Interactions have been observed when other nondepolarizing muscle relaxants have been administered in succession.
7.8 Procainamide
Procainamide has been shown to increase the duration of neuromuscular block and decrease infusion requirements of neuromuscular blocking agents [see Warnings and Precautions (5.10)].
7.9 Propofol
The use of propofol for induction and maintenance of anesthesia does not alter the clinical duration or recovery characteristics following recommended doses of rocuronium bromide.
7.10 Quinidine
Injection of quinidine during recovery from use of muscle relaxants is associated with recurrent paralysis. This possibility must also be considered for rocuronium bromide [see Warnings and Precautions (5.10)].
7.11 Succinylcholine
The use of rocuronium bromide before succinylcholine, for the purpose of attenuating some of the side effects of succinylcholine, has not been studied.
If rocuronium bromide is administered following administration of succinylcholine, it should not be given until recovery from succinylcholine has been observed. The median duration of action of rocuronium bromide 0.6 mg/kg administered after a 1 mg/kg dose of succinylcholine when T1 returned to 75% of control was 36 minutes (range: 14 to 57, n=12) vs. 28 minutes (range:17 to 51, n=12) without succinylcholine.
Use in Specific Populations
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Developmental toxicology studies have been performed with rocuronium bromide in pregnant, conscious, nonventilated rabbits and rats. Inhibition of neuromuscular function was the endpoint for high-dose selection. The maximum tolerated dose served as the high dose and was administered intravenously three times a day to rats (0.3 mg/kg, 15% to 30% of human intubation dose of 0.6 to 1.2 mg/kg based on the body surface unit of mg/m2) from day 6 to 17 and to rabbits (0.02 mg/kg, 25% human dose) from day 6 to 18 of pregnancy. High-dose treatment caused acute symptoms of respiratory dysfunction due to the pharmacological activity of the drug. Teratogenicity was not observed in these animal species. The incidence of late embryonic death was increased at the high dose in rats, most likely due to oxygen deficiency. Therefore, this finding probably has no relevance for humans because immediate mechanical ventilation of the intubated patient will effectively prevent embryo-fetal hypoxia. However, there are no adequate and well-controlled studies in pregnant women. Rocuronium bromide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.2 Labor and Delivery
The use of rocuronium bromide in Cesarean section has been studied in a limited number of patients [see Clinical Studies (14.1)]. Rocuronium bromide is not recommended for rapid sequence induction in Cesarean section patients.
8.4 Pediatric Use
The use of rocuronium bromide has been studied in pediatric patients 3 months to 14 years of age under halothane anesthesia. Of the pediatric patients anesthetized with halothane who did not receive atropine for induction, about 80% experienced a transient increase (30% or greater) in heart rate after intubation. One of the 19 infants anesthetized with halothane and fentanyl who received atropine for induction experienced this magnitude of change [see Dosage and Administration (2.6) and Clinical Studies (14.3)].
Rocuronium bromide was also studied in pediatric patients up to 17 years of age, including neonates, under sevoflurane (induction) and isoflurane/nitrous oxide (maintenance) anesthesia. Onset time and clinical duration varied with dose, the age of the patient, and anesthetic technique. The overall analysis of ECG data in pediatric patients indicates that the concomitant use of rocuronium bromide with general anesthetic agents can prolong the QTc interval. The data also suggest that rocuronium bromide may increase heart rate. However, it was not possible to conclusively identify an effect of rocuronium bromide independent of that of anesthesia and other factors. Additionally, when examining plasma levels of rocuronium bromide in correlation to QTc interval prolongation, no relationship was observed [see Dosage and Administration (2.6), Warnings and Precautions (5.9), and Clinical Studies (14.3)].
Rocuronium bromide is not recommended for rapid sequence intubation in pediatric patients. Recommendations for use in pediatric patients are discussed in other sections [see Dosage and Administration (2.6) and Clinical Pharmacology (12.2)].
8.5 Geriatric Use
Rocuronium bromide was administered to 140 geriatric patients (65 years or greater) in US clinical trials and 128 geriatric patients in European clinical trials. The observed pharmacokinetic profile for geriatric patients (n=20) was similar to that for other adult surgical patients [see Clinical Pharmacology (12.3)]. Onset time and duration of action were slightly longer for geriatric patients (n=43) in clinical trials. Clinical experiences and recommendations for use in geriatric patients are discussed in other sections [see Dosage and Administration (2.6), Warnings and Precautions (5.5), Clinical Pharmacology (12.2), and Clinical Studies (14.2)].
8.6 Patients with Hepatic Impairment
Since rocuronium bromide is primarily excreted by the liver, it should be used with caution in patients with clinically significant hepatic impairment. Rocuronium bromide 0.6 mg/kg has been studied in a limited number of patients (n=9) with clinically significant hepatic impairment under steady-state isoflurane anesthesia. After rocuronium bromide 0.6 mg/kg, the median (range) clinical duration of 60 (35 to 166) minutes was moderately prolonged compared to 42 minutes in patients with normal hepatic function. The median recovery time of 53 minutes was also prolonged in patients with cirrhosis compared to 20 minutes in patients with normal hepatic function. Four of eight patients with cirrhosis, who received rocuronium bromide 0.6 mg/kg under opioid/nitrous oxide/oxygen anesthesia, did not achieve complete block. These findings are consistent with the increase in volume of distribution at steady state observed in patients with significant hepatic impairment [see Clinical Pharmacology (12.3)]. If used for rapid sequence induction in patients with ascites, an increased initial dosage may be necessary to assure complete block. Duration will be prolonged in these cases. The use of doses higher than 0.6 mg/kg has not been studied [see Dosage and Administration (2.6)].
8.7 Patients with Renal Impairment
Due to the limited role of the kidney in the excretion of rocuronium bromide, usual dosing guidelines should be followed. In patients with renal dysfunction, the duration of neuromuscular blockade was not prolonged; however, there was substantial individual variability (range: 22 to 90 minutes) [see Clinical Pharmacology (12.3)].
Overdosage
10 OVERDOSAGE
Overdosage with neuromuscular blocking agents may result in neuromuscular block beyond the time needed for surgery and anesthesia. The primary treatment is maintenance of a patent airway, controlled ventilation, and adequate sedation until recovery of normal neuromuscular function is assured. Once evidence of recovery from neuromuscular block is observed, further recovery may be facilitated by administration of an anticholinesterase agent in conjunction with an appropriate anticholinergic agent.
Reversal of Neuromuscular Blockade
Anticholinesterase agents should not be administered prior to the demonstration of some spontaneous recovery from neuromuscular blockade. The use of a nerve stimulator to document recovery is recommended.
Patients should be evaluated for adequate clinical evidence of neuromuscular recovery, e.g., 5‑second head lift, adequate phonation, ventilation, and upper airway patency. Ventilation must be supported while patients exhibit any signs of muscle weakness.
Recovery may be delayed in the presence of debilitation, carcinomatosis, and concomitant use of certain drugs which enhance neuromuscular blockade or separately cause respiratory depression. Under such circumstances the management is the same as that of prolonged neuromuscular blockade.
Description
11 DESCRIPTION
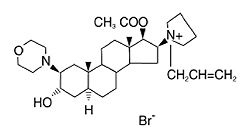
Rocuronium bromide injection is a nondepolarizing neuromuscular blocking agent with a rapid to intermediate onset depending on dose and intermediate duration. Rocuronium bromide is chemically designated as 1-[17β-(acetyloxy)-3α-hydroxy-2β-(4-morpholinyl)-5α-androstan-16β-yl]-1-(2-propenyl) pyrrolidinium bromide.
The structural formula is:
The chemical formula is C32H53BrN2O4 with a molecular weight of 609.70. The partition coefficient of rocuronium bromide in n-octanol/water is 0.5 at 20°C.
Rocuronium bromide is supplied as a sterile, nonpyrogenic, isotonic solution that is clear, colorless to yellow/orange, for intravenous injection only. Each mL contains 10 mg rocuronium bromide and 2 mg sodium acetate. The aqueous solution is adjusted to isotonicity with sodium chloride and to a pH of 4 with acetic acid and/or sodium hydroxide.
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Rocuronium bromide is a nondepolarizing neuromuscular blocking agent with a rapid to intermediate onset depending on dose and intermediate duration. It acts by competing for cholinergic receptors at the motor end-plate. This action is antagonized by acetylcholinesterase inhibitors, such as neostigmine and edrophonium.
12.2 Pharmacodynamics
The ED95 (dose required to produce 95% suppression of the first [T1] mechanomyographic [MMG] response of the adductor pollicis muscle [thumb] to indirect supramaximal train-of-four stimulation of the ulnar nerve) during opioid/nitrous oxide/oxygen anesthesia is approximately 0.3 mg/kg. Patient variability around the ED95 dose suggests that 50% of patients will exhibit T1 depression of 91% to 97%.
Table 4 presents intubating conditions in patients with intubation initiated at 60 to 70 seconds.
| * Excludes patients undergoing Cesarean section ** Pediatric patients were under halothane anesthesia Excellent intubating conditions = jaw relaxed, vocal cords apart and immobile, no diaphragmatic movement Good intubating conditions = same as excellent but with some diaphragmatic movement | ||
Rocuronium Bromide | Percent of Patients | Time to Completion of |
Adults* 18 to 64 yrs 0.45 (n=43) 0.6 (n=51) | 86% 96% | 1.6 (1.0 to 7.0) 1.6 (1.0 to 3.2) |
Infants** 3 mo to 1 yr 0.6 (n=18) Pediatric** 1 to 12 yrs 0.6 (n=12) | 100%
100% | 1.0 (1.0 to 1.5)
1.0 (0.5 to 2.3) |
Table 5 presents the time to onset and clinical duration for the initial dose of rocuronium bromide injection under opioid/nitrous oxide/oxygen anesthesia in adults and geriatric patients, and under halothane anesthesia in pediatric patients.
| n = the number of patients who had time to maximum block recorded Clinical duration = time until return to 25% of control T1. Patients receiving doses of 0.45 mg/kg who achieved less than 90% block (16% of these patients) had about 12 to 15 minutes to 25% recovery. | ||||||
Rocuronium Bromide | Time to ≥80% | Time to | Clinical Duration | |||
Adults 18 to 64 yrs |
|
|
| |||
0.45 (n=50) | 1.3 (0.8 to 6.2) | 3.0 (1.3 to 8.2) | 22 (12 to 31) | |||
0.6 (n=142) | 1.0 (0.4 to 6.0) | 1.8 (0.6 to 13.0) | 31 (15 to 85) | |||
0.9 (n=20) | 1.1 (0.3 to 3.8) | 1.4 (0.8 to 6.2) | 58 (27 to 111) | |||
1.2 (n=18) | 0.7 (0.4 to 1.7) | 1.0 (0.6 to 4.7) | 67 (38 to 160) | |||
Geriatric ≥65 yrs |
|
|
| |||
0.6 (n=31) | 2.3 (1.0 to 8.3) | 3.7 (1.3 to 11.3) | 46 (22 to 73) | |||
0.9 (n=5) | 2.0 (1.0 to 3.0) | 2.5 (1.2 to 5.0) | 62 (49 to 75) | |||
1.2 (n=7) | 1.0 (0.8 to 3.5) | 1.3 (1.2 to 4.7) | 94 (64 to 138) | |||
Infants 3 mo to 1 yr |
|
|
| |||
0.6 (n=17) | — | 0.8 (0.3 to 3.0) | 41 (24 to 68) | |||
0.8 (n=9) | — | 0.7 (0.5 to 0.8) | 40 (27 to 70) | |||
Pediatric 1 to 12 yrs |
|
|
| |||
0.6 (n=27) | 0.8 (0.4 to 2.0) | 1.0 (0.5 to 3.3) | 26 (17 to 39) | |||
0.8 (n=18) | — | 0.5 (0.3 to 1.0) | 30 (17 to 56) | |||
Table 6 presents the time to onset and clinical duration for the initial dose of Rocuronium Bromide Injection under sevoflurane (induction) and isoflurane/nitrous oxide (maintenance) anesthesia in pediatric patients.
| n=the number of patients with the highest number of observations for time to maximum block or reappearance T3. | |||||||||
Rocuronium Bromide | Time to | Time to | |||||||
Neonates birth to <28 days | 1.1 (0.6-2.2) | 40.3 (32.5-62.6) | |||||||
Infants 28 days to ≤3 mo | 0.5 (0.4-1.3) | 49.1 (13.5-79.9) | |||||||
Toddlers >3 mo to ≤2 yrs | 0.8 (0.3-1.9) | 39.2 (16.9-59.4) | |||||||
Children >2 yrs to ≤11 yrs | 0.9 (0.4-1.9) | 21.5 (17.5-38.0) | |||||||
Adolescents >11 to ≤17 yrs | 1.0 (0.5-1.7) | 37.5 (18.3-65.7) | |||||||
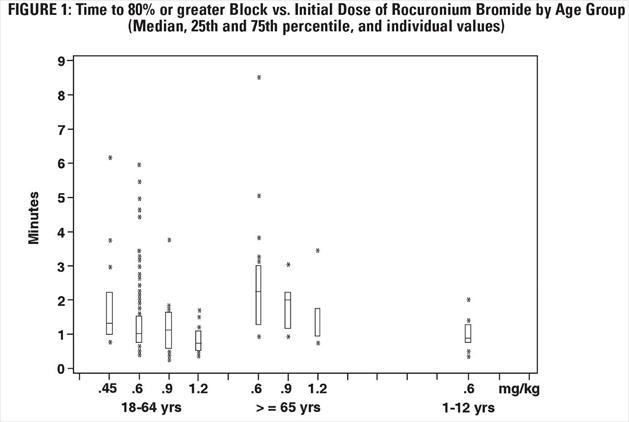
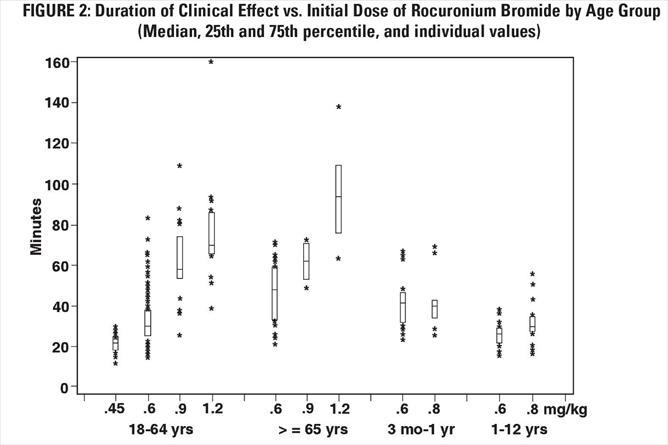
The time to 80% or greater block and clinical duration as a function of dose are presented in Figures 1 and 2.
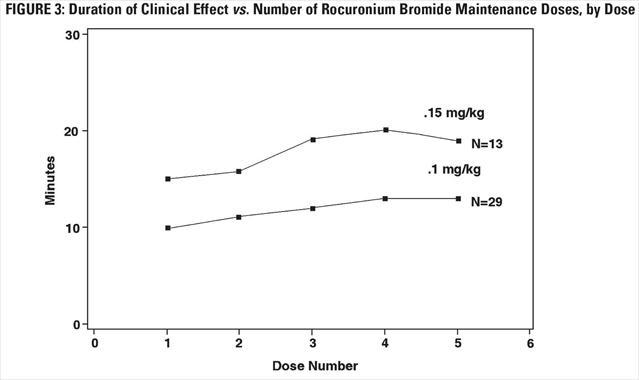
The clinical durations for the first five maintenance doses, in patients receiving five or more maintenance doses are represented in Figure 3 [see Dosage and Administration (2.4)].
Once spontaneous recovery has reached 25% of control T1, the neuromuscular block produced by rocuronium bromide is readily reversed with anticholinesterase agents, e.g., edrophonium or neostigmine.
The median spontaneous recovery from 25% to 75% T1 was 13 minutes in adult patients. When neuromuscular block was reversed in 36 adults at a T1 of 22% to 27%, recovery to a T1 of 89 (50 to 132)% and T4/T1 of 69 (38 to 92)% was achieved within 5 minutes. Only five of 320 adults reversed received an additional dose of reversal agent. The median (range) dose of neostigmine was 0.04 (0.01 to 0.09) mg/kg and the median (range) dose of edrophonium was 0.5 (0.3 to 1.0) mg/kg.
In geriatric patients (n=51) reversed with neostigmine, the median T4/T1 increased from 40% to 88% in 5 minutes.
In clinical trials with halothane, pediatric patients (n=27) who received 0.5 mg/kg edrophonium had increases in the median T4/T1 from 37% at reversal to 93% after 2 minutes. Pediatric patients (n=58) who received 1 mg/kg edrophonium had increases in the median T4/T1 from 72% at reversal to 100% after 2 minutes. Infants (n=10) who were reversed with 0.03 mg/kg neostigmine recovered from 25% to 75% T1 within 4 minutes.
There were no reports of less than satisfactory clinical recovery of neuromuscular function.
The neuromuscular blocking action of rocuronium bromide may be enhanced in the presence of potent inhalation anesthetics [see Drug Interactions (7.3)].
Hemodynamics
There were no dose-related effects on the incidence of changes from baseline (30% or greater) in mean arterial blood pressure (MAP) or heart rate associated with rocuronium bromide administration over the dose range of 0.12 to 1.2 mg/kg (4 x ED95) within 5 minutes after rocuronium bromide administration and prior to intubation. Increases or decreases in MAP were observed in 2% to 5% of geriatric and other adult patients, and in about 1% of pediatric patients. Heart rate changes (30% or greater) occurred in 0% to 2% of geriatric and other adult patients. Tachycardia (30% or greater) occurred in 12 of 127 pediatric patients. Most of the pediatric patients developing tachycardia were from a single study where the patients were anesthetized with halothane and who did not receive atropine for induction [see Clinical Studies (14.3)]. In US studies, laryngoscopy and tracheal intubation following rocuronium bromide administration were accompanied by transient tachycardia (30% or greater increases) in about one-third of adult patients under opioid/nitrous oxide/oxygen anesthesia. Animal studies have indicated that the ratio of vagal:neuromuscular block following rocuronium bromide administration is less than vecuronium but greater than pancuronium. The tachycardia observed in some patients may result from this vagal blocking activity.
Histamine Release
In studies of histamine release, clinically significant concentrations of plasma histamine occurred in 1 of 88 patients. Clinical signs of histamine release (flushing, rash, or bronchospasm) associated with the administration of rocuronium bromide were assessed in clinical trials and reported in 9 of 1137 (0.8%) patients.
12.3 Pharmacokinetics
Adult and Geriatric Patients
In an effort to maximize the information gathered in the in vivo pharmacokinetic studies, the data from the studies was used to develop population estimates of the parameters for the subpopulations represented (e.g., geriatric, pediatric, renal, and hepatic impairment). These population-based estimates and a measure of the estimate variability are contained in the following section.
Following intravenous administration of rocuronium bromide, plasma levels of rocuronium follow a three-compartment open model. The rapid distribution half-life is 1 to 2 minutes and the slower distribution half-life is 14 to 18 minutes. Rocuronium is approximately 30% bound to human plasma proteins. In geriatric and other adult surgical patients undergoing either opioid/nitrous oxide/oxygen or inhalational anesthesia, the observed pharmacokinetic profile was essentially unchanged [see Dosage and Administration (2.6)].
PK Parameters | Adults | Geriatrics |
Clearance (L/kg/hr) | 0.25 (0.08) | 0.21 (0.06) |
Volume of Distribution at Steady State (L/kg) | 0.25 (0.04) | 0.22 (0.03) |
t1/2 β Elimination (hr) | 1.4 (0.4) | 1.5 (0.4) |
In general, studies with normal adult subjects did not reveal any differences in the pharmacokinetics of rocuronium due to gender.
Studies of distribution, metabolism, and excretion in cats and dogs indicate that rocuronium is eliminated primarily by the liver. The rocuronium analog 17-desacetyl-rocuronium, a metabolite, has been rarely observed in the plasma or urine of humans administered single doses of 0.5 to 1 mg/kg with or without a subsequent infusion (for up to 12 hr) of rocuronium. In the cat, 17-desacetyl-rocuronium has approximately one-twentieth the neuromuscular blocking potency of rocuronium. The effects of renal failure and hepatic disease on the pharmacokinetics and pharmacodynamics of rocuronium in humans are consistent with these findings.
In general, patients undergoing cadaver kidney transplant have a small reduction in clearance which is offset pharmacokinetically by a corresponding increase in volume, such that the net effect is an unchanged plasma half-life. Patients with demonstrated liver cirrhosis have a marked increase in their volume of distribution resulting in a plasma half-life approximately twice that of patients with normal hepatic function. Table 8 shows the pharmacokinetic parameters in subjects with either impaired renal or hepatic function.
| * Differences in the calculated t1/2 β and Cl between this study and the study in young adults vs. geriatrics (≥65 years) is related to the different sample populations and anesthetic techniques. | ||||||
PK Parameters | Normal Renal and Hepatic Function | Renal Transplant Patients | Hepatic Dysfunction Patients | |||
Clearance (L/kg/hr) | 0.16 (0.05)* | 0.13 (0.04) | 0.13 (0.06) | |||
Volume of | 0.26 (0.03) | 0.34 (0.11) | 0.53 (0.14) | |||
t1/2 β Elimination (hr) | 2.4 (0.8)* | 2.4 (1.1) | 4.3 (2.6) | |||
The net result of these findings is that subjects with renal failure have clinical durations that are similar to but somewhat more variable than the duration that one would expect in subjects with normal renal function. Hepatically impaired patients, due to the large increase in volume, may demonstrate clinical durations approaching 1.5 times that of subjects with normal hepatic function. In both populations the clinician should individualize the dose to the needs of the patient [see Dosage and Administration (2.6)].
Tissue redistribution accounts for most (about 80%) of the initial amount of rocuronium administered. As tissue compartments fill with continued dosing (4 to 8 hours), less drug is redistributed away from the site of action and, for an infusion-only dose, the rate to maintain neuromuscular blockade falls to about 20% of the initial infusion rate. The use of a loading dose and a smaller infusion rate reduces the need for adjustment of dose.
Pediatric Patients
Under halothane anesthesia, the clinical duration of effects of rocuronium bromide did not vary with age in patients 4 months to 8 years of age. The terminal half-life and other pharmacokinetic parameters of rocuronium in these pediatric patients are presented in Table 9.
PK Parameters | Patient Age Range | ||
3 to <12 mos | 1 to <3 yrs | 3 to <8 yrs | |
Clearance (L/kg/hr) | 0.35 (0.08) | 0.32 (0.07) | 0.44 (0.16) |
Volume of | 0.30 (0.04) | 0.26 (0.06) | 0.21 (0.03) |
t1/2 β Elimination (hr) | 1.3 (0.5) | 1.1 (0.7) | 0.8 (0.3) |
Pharmacokinetics of rocuronium bromide were evaluated using a population analysis of the pooled pharmacokinetic datasets from 2 trials under sevoflurane (induction) and isoflurane/nitrous oxide (maintenance) anesthesia. All pharmacokinetic parameters were found to be linearly proportional to body weight. In patients under the age of 18 years clearance (CL) and volume of distribution (Vss) increase with bodyweight (kg) and age (years). As a result the terminal half-life of rocuronium bromide decreases with increasing age from 1.1 hour to 0.7-0.8 hour. Table 10 presents the pharmacokinetic parameters in the different age groups in the studies with sevoflurane (induction) and isoflurane/nitrous oxide (maintenance) anesthesia.
PK Parameters | Patient Age Range | ||||
Birth to | 28 days to | 3 mos to | 2 to ≤11 yrs | 11 to ≤17 yrs | |
CL (L/kg/hr) | 0.31 (0.07) | 0.30 (0.08) | 0.33 (0.10) | 0.35 (0.09) | 0.29 (0.14) |
Volume of Distribution (L/kg) | 0.42 (0.06) | 0.31 (0.03) | 0.23 (0.03) | 0.18 (0.02) | 0.18 (0.01) |
t1/2 β (hr) | 1.1 (0.2) | 0.9 (0.3) | 0.8 (0.2) | 0.7 (0.2) | 0.8 (0.3) |
Nonclinical Toxicology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in animals have not been performed with rocuronium bromide to evaluate carcinogenic potential or impairment of fertility. Mutagenicity studies (Ames test, analysis of chromosomal aberrations in mammalian cells, and micronucleus test) conducted with rocuronium bromide did not suggest mutagenic potential.
Clinical Studies
14 CLINICAL STUDIES
In US clinical studies, a total of 1137 patients received rocuronium bromide, including 176 pediatric, 140 geriatric, 55 obstetric, and 766 other adults. Most patients (90%) were ASA physical status I or II, about 9% were ASA III, and 10 patients (undergoing coronary artery bypass grafting or valvular surgery) were ASA IV. In European clinical studies, a total of 1394 patients received rocuronium bromide, including 52 pediatric, 128 geriatric (65 years or greater), and 1214 other adults.
14.1 Adult Patients
Intubation using doses of rocuronium bromide 0.6 to 0.85 mg/kg was evaluated in 203 adults in 11 clinical studies. Excellent to good intubating conditions were generally achieved within 2 minutes and maximum block occurred within 3 minutes in most patients. Doses within this range provide clinical relaxation for a median (range) time of 33 (14 to 85) minutes under opioid/nitrous oxide/oxygen anesthesia. Larger doses (0.9 and 1.2 mg/kg) were evaluated in two studies with 19 and 16 patients under opioid/nitrous oxide/oxygen anesthesia and provided 58 (27 to 111) and 67 (38 to 160) minutes of clinical relaxation, respectively.
Cardiovascular Disease
In one clinical study, 10 patients with clinically significant cardiovascular disease undergoing coronary artery bypass graft received an initial dose of 0.6 mg/kg rocuronium bromide. Neuromuscular block was maintained during surgery with bolus maintenance doses of 0.3 mg/kg. Following induction, continuous 8 mcg/kg/min infusion of rocuronium bromide produced relaxation sufficient to support mechanical ventilation for 6 to 12 hours in the surgical intensive care unit (SICU) while the patients were recovering from surgery.
Rapid Sequence Intubation
Intubation was assessed in patients in 6 clinical studies where anesthesia was induced with either thiopental (3 to 6 mg/kg) or propofol (1.5 to 2.5 mg/kg) in combination with either fentanyl (2 to 5 mcg/kg) or alfentanil (1 mg). Most of the patients also received a premedication such as midazolam or temazepam. Most patients had intubation attempted within 60 to 90 seconds of administration of rocuronium bromide 0.6 mg/kg or succinylcholine 1 to 1.5 mg/kg. Excellent or good intubating conditions were achieved in 119/120 (99% [95% confidence interval 95% to 99.9%]) patients receiving rocuronium bromide and in 108/110 (98% [94% to 99.8%]) patients receiving succinylcholine. The duration of action of rocuronium bromide 0.6 mg/kg is longer than succinylcholine and at this dose is approximately equivalent to the duration of other intermediate-acting neuromuscular blocking drugs.
Obese Patients
Rocuronium bromide was dosed according to actual body weight (ABW) in most clinical studies. The administration of rocuronium bromide in the 47 of 330 (14%) patients who were at least 30% or more above their ideal body weight (IBW) was not associated with clinically significant differences in the onset, duration, recovery, or reversal of rocuronium bromide-induced neuromuscular block.
In one clinical study in obese patients, rocuronium bromide 0.6 mg/kg was dosed according to ABW (n=12) or IBW (n=11). Obese patients dosed according to IBW had a longer time to maximum block, a shorter median (range) clinical duration of 25 (14 to 29) minutes, and did not achieve intubating conditions comparable to those dosed based on ABW. These results support the recommendation that obese patients be dosed based on actual body weight [see Dosage and Administration (2.6)].
Obstetric Patients
Rocuronium bromide 0.6 mg/kg was administered with thiopental, 3 to 4 mg/kg (n=13) or 4 to 6 mg/kg (n=42), for rapid sequence induction of anesthesia for Cesarean section. No neonate had APGAR scores greater than 7 at 5 minutes. The umbilical venous plasma concentrations were 18% of maternal concentrations at delivery. Intubating conditions were poor or inadequate in 5 of 13 women receiving 3 to 4 mg/kg thiopental when intubation was attempted 60 seconds after drug injection. Therefore, rocuronium bromide is not recommended for rapid sequence induction in Cesarean section patients.
14.2 Geriatric Patients
Rocuronium bromide was evaluated in 55 geriatric patients (ages 65 to 80 years) in six clinical studies. Doses of 0.6 mg/kg provided excellent to good intubating conditions in a median (range) time of 2.3 (1 to 8) minutes. Recovery times from 25% to 75% after these doses were not prolonged in geriatric patients compared to other adult patients [see Dosage and Administration (2.6) and Use in Specific Populations (8.5)].
14.3 Pediatric Patients
Rocuronium bromide 0.45, 0.6, or 1 mg/kg was evaluated under sevoflurane (induction) and isoflurane/nitrous oxide (maintenance) anesthesia for intubation in 326 patients in 2 studies. In 1 of these studies maintenance bolus and infusion requirements were evaluated in 137 patients. In all age groups, doses of 0.6 mg/kg provided time to maximum block in about 1 minute. Across all age groups, median (range) time to reappearance of T3 for doses of 0.6 mg/kg was shortest in the children [36.7 (20.1-65.9) minutes] and longest in infants [59.8 (32.3-87.8) minutes]. For pediatric patients older than 3 months, the time to recovery was shorter after stopping infusion maintenance when compared with bolus maintenance [see Dosage and Administration (2.6) and Use in Specific Populations (8.4)].
Rocuronium bromide 0.6 or 0.8 mg/kg was evaluated for intubation in 75 pediatric patients (n=28; age 3 to 12 months, n=47; age 1 to 12 years) in three studies using halothane (1% to 5%) and nitrous oxide (60% to 70%) in oxygen. Doses of 0.6 mg/kg provided a median (range) time to maximum block of 1 (0.5 to 3.3) minute(s). This dose provided a median (range) time of clinical relaxation of 41 (24 to 68) minutes in 3 month to 1 year-old infants and 26 (17 to 39) minutes in 1 to 12 year-old pediatric patients [see Dosage and Administration (2.6) and Use in Specific Populations (8.4)].
How Supplied/Storage and Handling
16 HOW SUPPLIED/STORAGE AND HANDLING
Rocuronium bromide is available in the following:
| Unit of Sale | Concentration |
|---|---|
| NDC 0409-9558-05 Box of 10 Multiple Dose Vials | 50 mg/5 mL (10 mg/mL) |
| NDC 0409-9558-10 Box of 10 Multiple Dose Vials | 100 mg/10 mL (10 mg/mL) |
This container closure is not made with natural rubber latex.
Rocuronium bromide should be stored in a refrigerator, 2° to 8°C (36° to 46°F). DO NOT FREEZE. Upon removal from refrigeration to room temperature storage conditions (25°C/77°F), use rocuronium bromide within 60 days. Use opened vials of rocuronium bromide within 30 days.
Safety and Handling
There is no specific work exposure limit for rocuronium bromide. In case of eye contact, flush with water for at least 10 minutes.
Medication Guide
17 PATIENT COUNSELING INFORMATION
Obtain information about your patient's medical history, current medications, any history of hypersensitivity to rocuronium bromide or other neuromuscular blocking agents. If applicable, inform your patients that certain medical conditions and medications might influence how rocuronium bromide works.
In addition, inform your patient that severe anaphylactic reactions to neuromuscular blocking agents, including rocuronium bromide, have been reported. Since allergic cross-reactivity has been reported in this class, request information from your patients about previous anaphylactic reactions to other neuromuscular blocking agents.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA 
LAB-1239-4.0
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information. 9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.