NIVESTYM™
(filgrastim-aafi)
Find NIVESTYM™ medical information:
Find NIVESTYM™ medical information:
NIVESTYM™ Quick Finder
What is NIVESTYM?
What is NIVESTYM?
NIVESTYM is a man-made form of granulocyte colony-stimulating factor (G-CSF). G-CSF is a substance produced by the body. It stimulates the growth of neutrophils, a type of white blood cell important in the body's fight against infection.
Do not take NIVESTYM if you have had a serious allergic reaction to human G-CSFs such as filgrastim products or pegfilgrastim products.
Before you take NIVESTYM, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have a sickle cell disorder.
- •
- have kidney problems.
- •
- are receiving radiation therapy.
- •
- are pregnant or plan to become pregnant. It is not known if NIVESTYM will harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if NIVESTYM passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive NIVESTYM?
How will I receive NIVESTYM?
- •
- NIVESTYM injections can be given by a healthcare provider by intravenous (IV) infusion or under your skin (subcutaneous injection). Your healthcare provider may decide subcutaneous injections can be given at home by you or your caregiver. If NIVESTYM is given at home, see the detailed "Instructions for Use" that comes with your NIVESTYM for information on how to prepare and inject a dose of NIVESTYM.
- •
- You and your caregiver should be shown how to prepare and inject NIVESTYM before you use it, by your healthcare provider.
- •
- You should not try to inject a dose of NIVESTYM less than 0.3 mL (180 mcg) from a NIVESTYM prefilled syringe. A dose less than 0.3 mL cannot be accurately measured using the NIVESTYM prefilled syringe.
- •
- Your healthcare provider will tell you how much NIVESTYM to inject and when to inject it. Do not change your dose or stop NIVESTYM unless your healthcare provider tells you to.
- •
- If you are receiving NIVESTYM because you are also receiving chemotherapy, your dose of NIVESTYM should be injected at least 24 hours before or 24 hours after your dose of chemotherapy. Your healthcare provider will do blood tests to monitor your white blood cell count, and if necessary, adjust your NIVESTYM dose.
- •
- If you miss a dose of NIVESTYM, talk to your healthcare provider about when you should give your next dose.
What are the possible side effects of NIVESTYM?
What are the possible side effects of NIVESTYM?
NIVESTYM may cause serious side effects, including:
- •
- Spleen rupture. Your spleen may become enlarged and can rupture. A ruptured spleen can cause death. Call your healthcare provider right away if you have pain in the left upper stomach (abdomen) area or your left shoulder.
- •
- A serious lung problem called acute respiratory distress syndrome (ARDS). Call your healthcare provider or get emergency medical help right away if you have shortness of breath with or without a fever, trouble breathing, or a fast rate of breathing.
- •
- Serious allergic reactions. NIVESTYM can cause serious allergic reactions. These reactions can cause a rash over your whole body, shortness of breath, wheezing, dizziness, swelling around your mouth or eyes, fast heart rate, and sweating. If you have any of these symptoms, stop using NIVESTYM and call your healthcare provider or get emergency medical help right away.
- •
- Sickle cell crises. You may have a serious sickle cell crisis, which could lead to death, if you have a sickle cell disorder and receive NIVESTYM. Call your healthcare provider right away if you have symptoms of sickle cell crisis such as pain or difficulty breathing.
- •
- Kidney injury (glomerulonephritis). NIVESTYM can cause kidney injury. Call your healthcare provider right away if you develop any of the following symptoms:
- o
- swelling of your face or ankles
- o
- blood in your urine or dark colored urine
- o
- you urinate less than usual
- •
- Capillary leak syndrome. NIVESTYM can cause fluid to leak from blood vessels into your body's tissues. This condition is called "Capillary Leak Syndrome" (CLS). CLS can quickly cause you to have symptoms that may become life-threatening. Get emergency medical help right away if you develop any of the following symptoms:
- o
- swelling or puffiness and are urinating less than usual
- o
- trouble breathing
- o
- swelling of your stomach area (abdomen) and feeling of fullness
- o
- dizziness or feeling faint
- o
- a general feeling of tiredness
- •
- Myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
- o
- NIVESTYM may increase the risk of developing a precancerous condition called MDS or a type of blood cancer called AML in people who were born with low white blood cell counts (congenital neutropenia).
- o
- If you have breast cancer or lung cancer, when NIVESTYM is used with chemotherapy and radiation therapy, or with radiation therapy only, you may have an increased risk of developing MDS or AML.
- o
- Symptoms of MDS and AML may include tiredness, fever, and easy bruising or bleeding.
- o
- Call your healthcare provider if you develop any of these symptoms during treatment with NIVESTYM.
- •
- Decreased platelet count (thrombocytopenia). Your healthcare provider will check your blood during treatment with NIVESTYM. Tell your healthcare provider if you have unusual bleeding or bruising during treatment with NIVESTYM. This could be a sign of decreased platelet counts, which may reduce the ability of your blood to clot.
- •
- Increased white blood cell count (leukocytosis). Your healthcare provider will check your blood during treatment with NIVESTYM.
- •
- Inflammation of your blood vessels (cutaneous vasculitis). Tell your healthcare provider right away if you develop purple spots or redness of your skin.
- •
- Inflammation of the aorta (aortitis). Inflammation of the aorta (the large blood vessel which transports blood from the heart to the body) has been reported in patients who received NIVESTYM. Symptoms may include fever, abdominal pain, feeling tired, and back pain. Call your healthcare provider if you experience these symptoms.
The most common side effects experienced in patients receiving NIVESTYM include:
- •
- Patients with cancer receiving chemotherapy: fever, pain, rash, cough, and shortness of breath
- •
- Patients with acute myeloid leukemia receiving chemotherapy: pain, nose bleed, and rash
- •
- Patients with cancer receiving chemotherapy followed by bone marrow transplant: rash
- •
- Patients who are having their own blood cells collected: bone pain, fever, and headache
- •
- Patients with severe chronic neutropenia: pain, decreased red blood cells, nose bleed, diarrhea, reduced sensation, and hair loss
These are not all the possible side effects of NIVESTYM. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store NIVESTYM?
How should I store NIVESTYM?
- •
- Store NIVESTYM in the refrigerator between 36°F to 46°F (2°C to 8°C).
- •
- Do not freeze.
- •
- Keep NIVESTYM in the original carton to protect from light or physical damage. Do not leave NIVESTYM in direct sunlight.
- •
- Do not shake NIVESTYM.
- •
- Take NIVESTYM out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- •
- Throw away (dispose of) any NIVESTYM that has been left at room temperature for longer than 24 hours.
- •
- After you inject your dose, throw away (dispose of) any unused NIVESTYM left in the vials or prefilled syringes. Do not save unused NIVESTYM in the vials or prefilled syringes for later use.
Keep NIVESTYM out of the reach of children.
General information about the safe and effective use of NIVESTYM.
General information about the safe and effective use of NIVESTYM.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NIVESTYM for a condition for which it was not prescribed. Do not give NIVESTYM to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about NIVESTYM that is written for healthcare professionals.
What are the ingredients in NIVESTYM?
What are the ingredients in NIVESTYM?
Active ingredient: (filgrastim-aafi)
Inactive ingredients: acetate, polysorbate 80, sodium, sorbitol, and water for Injection
Manufactured by Hospira, Inc., a Pfizer Company, Lake Forest, IL 60045 USA
US License No. 1974
Distributed by Pfizer Labs, division of Pfizer Inc., New York, NY 10001 USA
LAB-0935-5.0
For more information go to www.pfizer.com or call 1-800-438-1985.

This Patient Information has been approved by the U.S. Food and Drug Administration Revised: March 2023
Instructions for Use
NIVESTYM (Neye-ves-tim)
(filgrastim-aafi)
injection
Single-Dose Prefilled Syringe
Important
Read the Patient Information for important information you need to know about NIVESTYM before using this Instructions for Use.
Before you use a NIVESTYM prefilled syringe, read this important information.
Storing your prefilled syringe |
- •
- Store the NIVESTYM prefilled syringe in the refrigerator between 36˚F to 46˚F (2˚C to 8˚C).
- •
- Do not freeze.
- •
- Keep the NIVESTYM prefilled syringe in the original carton to protect from light or physical damage.
- •
- Take the prefilled syringe out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- •
- The NIVESTYM prefilled syringe may be allowed to reach room temperature for up to 24 hours. Throw away (dispose of) any NIVESTYM prefilled syringe that has been left at room temperature for longer than 24 hours.
- •
- After you inject your dose, throw away (dispose of) any unused NIVESTYM left in the prefilled syringe. Do not save unused NIVESTYM in the prefilled syringe for later use.
- •
- Keep NIVESTYM and all medicines out of the reach of children.
Using your prefilled syringe |
- •
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
- •
- You should not inject a dose of NIVESTYM less than 0.3 mL (180 mcg) from a NIVESTYM prefilled syringe. A dose less than 0.3 mL cannot be accurately measured using the NIVESTYM prefilled syringe.
- •
- Make sure the name NIVESTYM appears on the carton and prefilled syringe label.
- •
- Do not use a NIVESTYM prefilled syringe after the expiration date on the label.
- •
- Do not shake the NIVESTYM prefilled syringe.
- •
- The prefilled syringe has a needle guard that needs to be activated to cover the needle after the injection is given. The needle guard will help prevent needle stick injuries to anyone who handles the prefilled syringe.
- •
- Do not remove the needle cover from the prefilled syringe until you are ready to inject.
- •
- Do not use the NIVESTYM prefilled syringe if the needle cover is missing.
- •
- Do not use the prefilled syringe if the carton is open or damaged.
- •
- Do not use a prefilled syringe if it has been dropped on a hard surface. The prefilled syringe may be broken even if you cannot see the break. Use a new prefilled syringe.
Call your healthcare provider if you have any questions.
About the NIVESTYM prefilled syringe | |
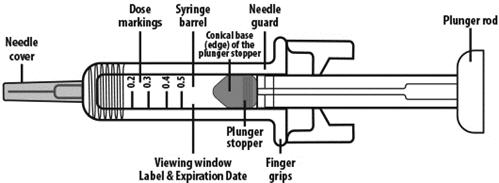
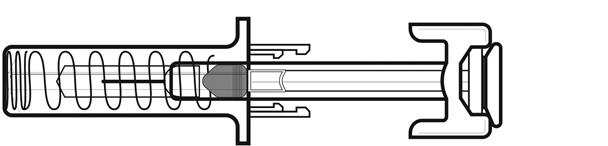
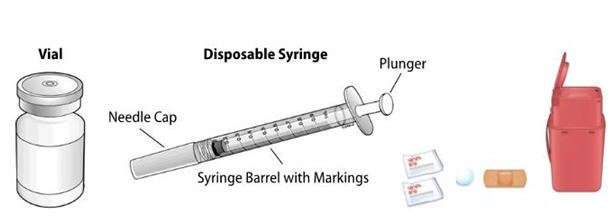
NIVESTYM prefilled syringe parts (see Figure A). NIVESTYM 300 mcg/0.5 mL prefilled syringe is shown as an example. | |
Figure A | |
What you need for your injection | |
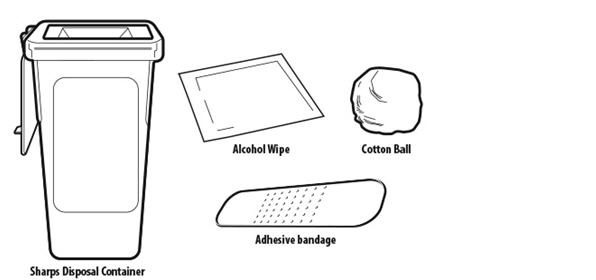
Included in the carton:
Not included in the carton (see Figure B)
| |
Figure B | |
Preparing the NIVESTYM prefilled syringe | |
Step 1: | Find a clean, well-lit flat work surface. |
Step 2: | Take the carton containing the NIVESTYM prefilled syringe out of the refrigerator and leave it unopened on your work surface for at least 30 minutes so that it reaches room temperature. Put the original carton with any unused prefilled syringes back in the refrigerator.
|
Step 3: | Wash your hands with soap and water. |
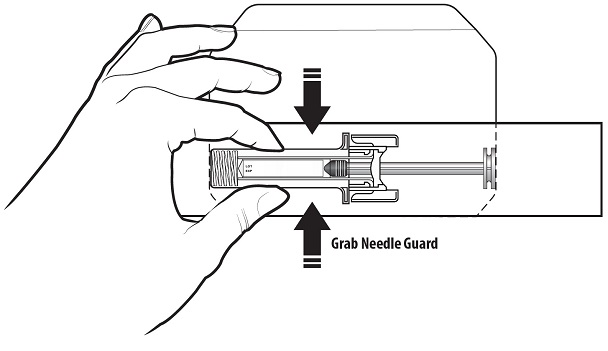
Step 4: | Remove the prefilled syringe from the carton by the needle guard only. Do not remove the prefilled syringe by its plunger or needle cover. See Figure C. Check to make sure that the needle guard is covering the barrel of the prefilled syringe. |
Figure C | |
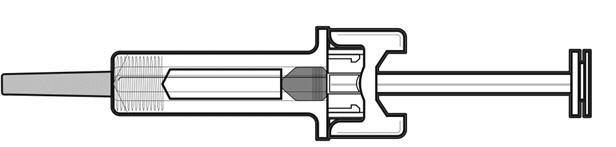
Do not push the needle guard over the needle cover before the injection. This may activate or lock the needle guard. See Figure D that shows a needle guard that has not yet been activated. This is how the prefilled syringe looks before use. | |
Figure D | |
If the needle guard is covering the needle that means it has been activated. See Figure E that shows a needle guard that has been activated. This is how the prefilled syringe looks after use. Do not use the NIVESTYM prefilled syringe. Get another prefilled syringe that has not been activated and is ready to use. | |
Figure E | |
Step 5: | Check the expiration date on the NIVESTYM prefilled syringe. Do not use the NIVESTYM prefilled syringe if the expiration date has passed. |
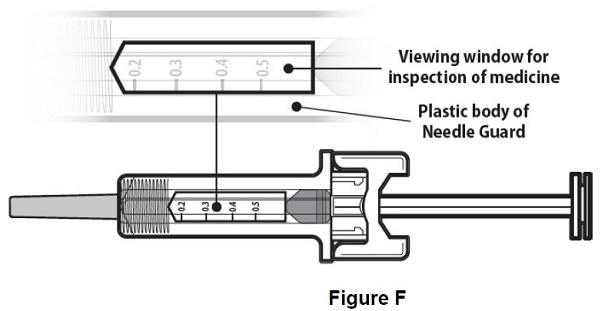
Step 6: | Inspect the medicine and prefilled syringe. Turn the prefilled syringe so you can see the medicine and markings in the window. Make sure that you look at the medicine only through the viewing window on the prefilled syringe (see Figure F). Do not inspect the medicine through the plastic of the needle guard. Make sure the medicine in the prefilled syringe is clear and colorless. |
Figure F | |
| |
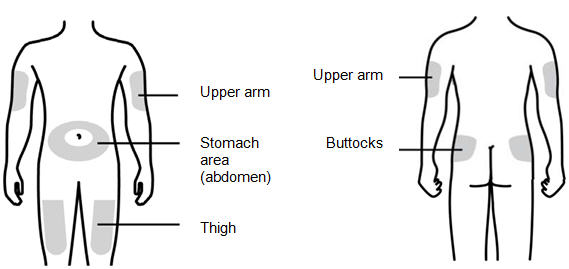
Step 7: | Choose the injection site
|
Figure G | |
| |
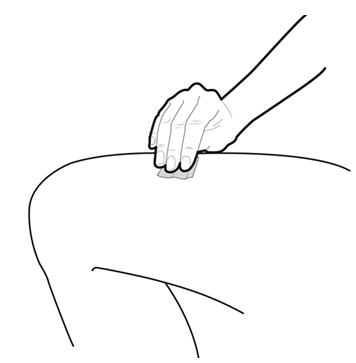
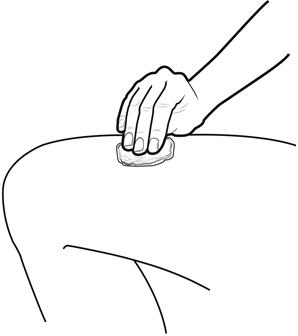
Step 8: | Clean your injection site with an alcohol wipe. See Figure H.
|
Figure H | |
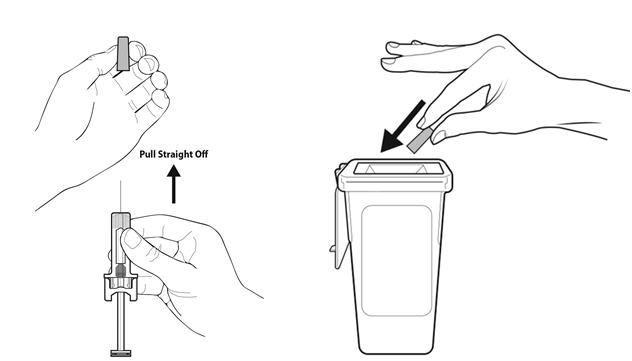
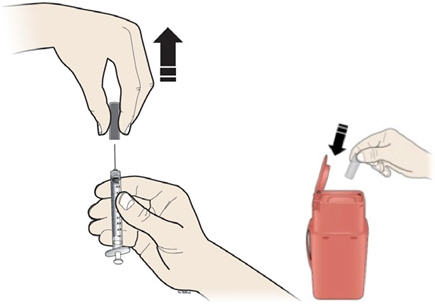
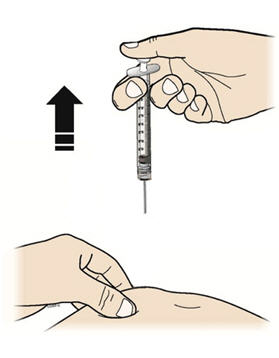
Step 9: | Hold the prefilled syringe by the needle guard with the needle cover pointing up. Carefully pull the needle cover straight off and away from your body. Throw away the needle cover. Do not recap the needle. See Figure I. |
Figure I | |
Your healthcare provider has prescribed either a "full" syringe dose or a "partial" syringe dose.
| |
Partial dosing | |
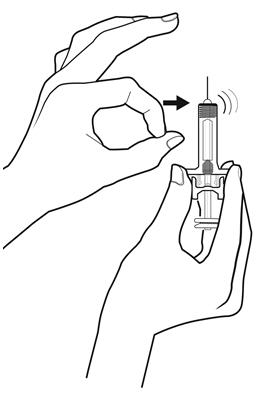
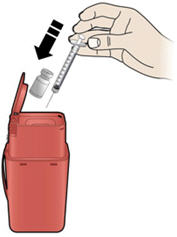
Step 10: | Point the needle up and tap gently until the air rises to the top. See Figure J. |
Figure J | |
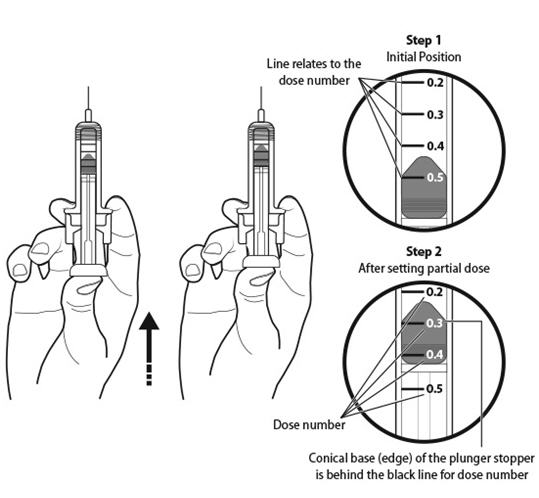
Step 11: | Holding the prefilled syringe as shown, slowly push up on the plunger rod to push out the extra air and medicine until the end of the conical base (edge) of the plunger stopper lines up with the syringe marking for your prescribed dose. See Figure K for an example of a dose of 0.3 mL. Your dose may be different than the example shown. |
Figure K | |
Giving the NIVESTYM prefilled syringe injection | |
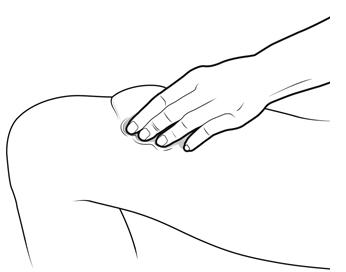
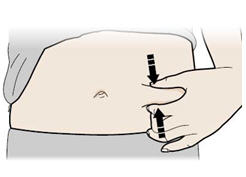
Step 12: | With one hand, gently pinch a fold of skin at the injection site. Hold the pinch. See Figure L. |
Figure L | |
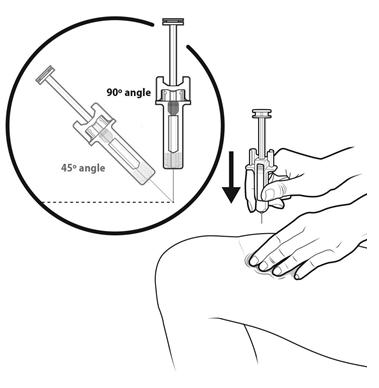
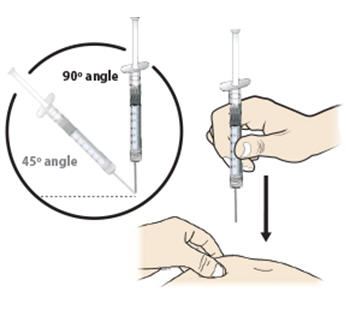
Step 13: | With your other hand, hold the prefilled syringe like you would hold a pencil. Use a quick "dart-like" motion to insert the needle at a 45 to 90 degree angle into the skin as shown. See Figure M. |
Figure M | |
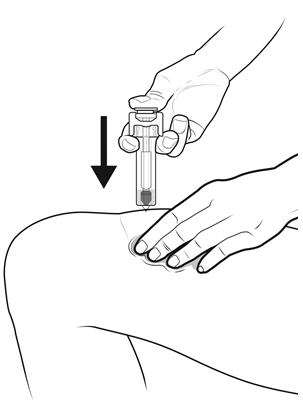
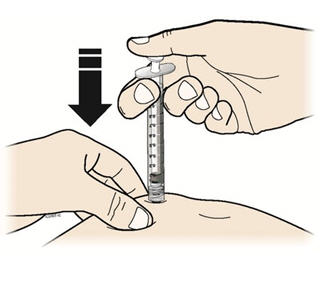
Step 14: | Using slow and constant pressure, press down on the plunger rod until it reaches the bottom. See Figure N. |
Figure N | |
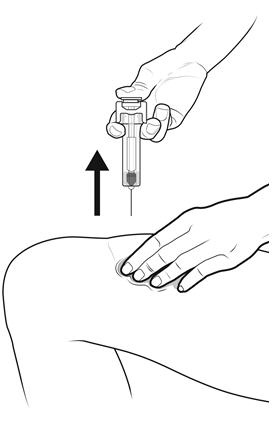
Step 15: | Keep the plunger rod fully pressed down while you carefully pull the needle straight out from the injection site. See Figure O. |
Figure O | |
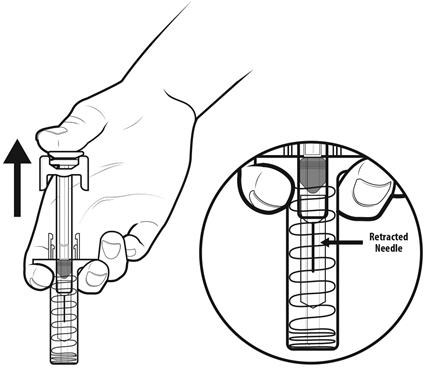
Step 16: | As you let go of the plunger rod, the needle guard will automatically slide over the needle until the needle is completely covered and the needle guard locks into place. Do not recap the needle. See Figure P. |
Figure P | |
Step 17: | There may be a small amount of blood at the injection site. You can press a cotton ball or gauze over the injection site and hold it for 10 seconds. Do not rub the injection site. You may cover the injection site with a small adhesive bandage, if needed. See Figure Q. |
Figure Q | |
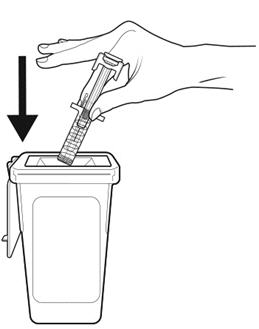
Step 18: | Throw away (dispose of) the syringe as instructed by your healthcare provider or by following the instructions below. See Figure R. |
Figure R | |
Disposing of (throw away) used NIVESTYM prefilled syringes | |
- •
- Put the used prefilled syringe in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) prefilled syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Hospira, Inc.,
a Pfizer Company
Lake Forest, IL 60045 USA
US License No. 1974
Distributed by Pfizer Labs,
division of Pfizer Inc.,
New York, NY 10001 USA
LAB-0938-6.0
For more information go to www.pfizer.com or call 1-800-438-1985.
Revised: February 2024
Instructions for Use
NIVESTYM (Neye-ves-tim)
(filgrastim-aafi)
injection
Single-Dose Vial
Important
Read the Patient Information for important information you need to know about NIVESTYM before using these Instructions for Use.
Before you use a NIVESTYM vial, read this important information:
Storing your NIVESTYM vial
- •
- Store the vial in the refrigerator between 36˚F to 46˚F (2˚C to 8˚C).
- •
- Do not freeze.
- •
- Keep the vial in the original carton to protect from light or physical damage.
- •
- Take the vial out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- •
- Throw away (dispose of) any vial that has been left at room temperature for longer than 24 hours.
- •
- After you inject your dose, throw away (dispose of) any unused NIVESTYM left in the vial. Do not save unused NIVESTYM in the vial for later use.
Keep NIVESTYM and all medicines out of the reach of children.
Using your vial
- •
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
- •
- Make sure the name NIVESTYM appears on the carton and vial label.
- •
- Only use the vial 1 time. Discard (throw away) the vial with any remaining NIVESTYM liquid.
- •
- Do not use a vial after the expiration date on the label.
- •
- Do not shake the vial.
- •
- Do not use the vial if the medicine is cloudy or discolored or contains flakes or particles.
Call your healthcare provider if you have any questions.
Step 1: Prepare
- A.
- Remove the vial from the refrigerator.
Find a clean, well-lit, flat work surface. Place the vial on your clean work surface for 30 minutes and allow it to reach room temperature before you give an injection.- •
- Do not try to warm the vial by using a heat source such as hot water or microwave.
- •
- Do not leave the vial in direct sunlight.
- •
- Do not shake the vial.
- •
- Use the vial only 1 time.
- B.
- Inspect the vial.
Make sure the medicine in the vial is clear and colorless.- •
- Do not use the vial if:
- o
- The medicine is cloudy or discolored or contains flakes or particles.
- o
- The expiration date printed on the label has passed.
- •
- In all cases, use a new vial and call your healthcare provider.
- C.
- Gather all materials needed for your injection.
Wash your hands thoroughly with soap and water. On your clean, well-lit, flat work surface, place:- •
- 1 Vial
- •
- 1 Disposable syringe and needle
- •
- 2 Alcohol wipes
- •
- 1 Cotton ball or gauze pad
- •
- 1 Adhesive bandage
- •
- Sharps disposal container
- •
- Only use the disposable syringes and needles that your healthcare provider prescribes.
- •
- Only use the syringes and needles 1 time. Throw away (dispose of) any used syringes and needles. See Step 5 Finish, for instructions about how to properly dispose of used syringes and needles.
- •
- You should only use a syringe that is marked in tenths of milliliters (mL).
- •
- Your healthcare provider will show you how to measure the correct dose of NIVESTYM. This dose will be measured in milliliters (mL).
Step 2: Get Ready
- D.
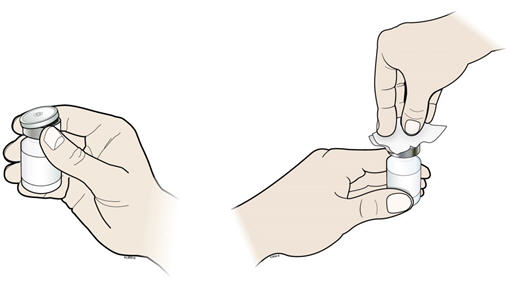
- Take the cap off the vial. Clean the rubber stopper with 1 alcohol wipe.
- E.
- Check the carton containing the needle and syringe. If the carton has been opened or damaged, do not use that needle and syringe. Dispose of (throw away) that needle and syringe in the sharps disposal container.
- F.
- Hold the syringe by the barrel with the needle cap pointing up. Carefully pull the needle cap straight off and away from your body.
Pull back on the plunger and draw air into the syringe that is the same amount (mL) as the dose of NIVESTYM that your healthcare provider prescribed.
Important: Throw away the needle cap into the sharps disposal container. Do not recap the needle.- G.
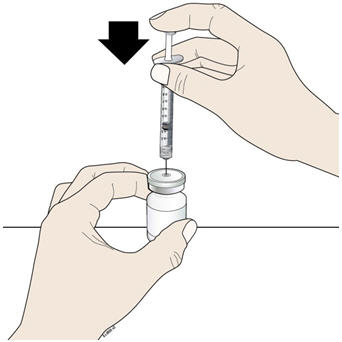
- Keep the vial on the flat work surface and insert the needle straight down through the rubber stopper. Do not insert the needle through the rubber stopper more than 1 time.
- H.
- Push the plunger down and inject all the air from the syringe into the vial of NIVESTYM.
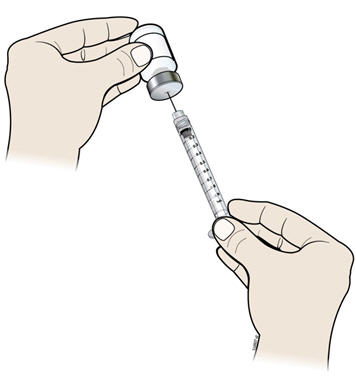
- I.
- Keep the needle in the vial and turn the vial upside down. Make sure that the NIVESTYM liquid is covering the tip of the needle.
- J.
- Keep the vial upside down and slowly pull back on the plunger to fill the syringe barrel with NIVESTYM to the correct marking amount (mL) of medicine that matches the dose your healthcare provider prescribed.
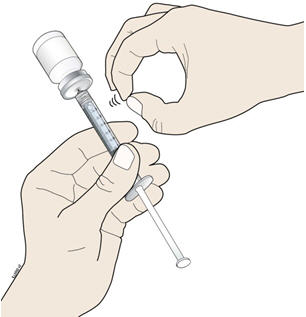
- K.
- Keep the needle in the vial and check for air bubbles in the syringe. If there are air bubbles, gently tap the syringe barrel with your finger until the air bubbles rise to the top. Slowly push the plunger up to push the air bubbles out of the syringe.
- L.
- Keep the tip of the needle in the liquid and again pull the plunger back to the number on the syringe barrel that matches your dose. Check again for air bubbles. The air in the syringe will not hurt you, but too large an air bubble can reduce your dose of NIVESTYM. If there are still air bubbles, repeat the steps above to remove them.
- M.
- Check again to make sure that you have the correct dose in the syringe. It is important that you use the exact dose prescribed by your healthcare provider. Do not remove the needle from the vial. Lay the vial down on its side with the needle still in the vial.
Step 3: Select and Prepare the Injection Site
- N.
- Prepare and clean your injection site.
You can use:
- •
- Thigh
- •
- Stomach area (abdomen), except for a 2-inch area right around your navel (belly button)
- •
- Upper outer area of your buttocks (only if someone else is giving you the injection)
- •
- Outer area of upper arm (only if someone else is giving you the injection)
Clean your injection site with a clean alcohol wipe.
Let your skin dry. - •
- Do not touch this area again before injecting.
- •
- If you want to use the same injection site, make sure it is not the same spot on the injection site area you used for a previous injection.
- •
- Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks.
Step 4: Subcutaneous (under the skin) injection
- O.
- Remove the prepared syringe and needle from the vial.
- P.
- Pinch your injection site to create a firm surface.
Important: Keep skin pinched while injecting. - Q.
- Hold the pinch. Insert the needle into the skin at a 45 to 90 degree angle.
- R.
- Using slow and constant pressure, push the plunger until it reaches the bottom.
When done gently pull the needle out of the injection site at the same 45 to 90 degree angle used to insert it.
- S.
- Dispose of (throw away) the used needle and syringe.
- •
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles, and syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- T.
- Examine the injection site.
If there is blood, press a cotton ball or gauze pad on your injection site. Do not rub the injection site. Apply an adhesive bandage if needed.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Hospira, Inc.,
a Pfizer Company
Lake Forest, IL 60045 USA
US License No. 1974
Distributed by Pfizer Labs,
division of Pfizer Inc.,
New York, NY 10001 USA
LAB-0937-5.0
For more information go to www.pfizer.com or call 1-800-438-1985.
Revised: August 2023
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Review the steps for direct patient administration with patients and caregivers. Training by the healthcare provider should aim to ensure that patients and caregivers can successfully perform all of the steps in the Instructions for Use of NIVESTYM vial and prefilled syringe, including showing the patient or caregiver how to measure the required dose, particularly if a patient is on a dose other than the entire prefilled syringe. If a patient or caregiver is not able to demonstrate that they can measure the dose and administer the product successfully, you should consider whether the patient is an appropriate candidate for self-administration of NIVESTYM or whether the patient would benefit from a different NIVESTYM presentation. Advise patients of the following risks and potential risks with NIVESTYM:
- •
- Rupture or enlargement of the spleen may occur. Symptoms include left upper quadrant abdominal pain or left shoulder pain. Advise patients to report pain in these areas to their physician immediately [see Warnings and Precautions (5.1)].
- •
- Dyspnea, with or without fever, progressing to Acute Respiratory Distress Syndrome, may occur.
Advise patients to report dyspnea to their physician immediately [see Warnings and Precautions (5.2)]. - •
- Serious allergic reactions may occur, which may be signaled by rash‚ facial edema‚ wheezing‚ dyspnea‚ hypotension‚ or tachycardia. Advise patients to seek immediate medical attention if signs or symptoms of hypersensitivity reaction occur [see Warnings and Precautions (5.3)].
- •
- In patients with sickle cell disease, sickle cell crisis and death have occurred. Discuss potential risks and benefits for patients with sickle cell disease prior to the administration of human granulocyte colony-stimulating factors [see Warnings and Precautions (5.4)].
- •
- Glomerulonephritis may occur. Symptoms include swelling of the face or ankles, dark colored urine or blood in the urine, or a decrease in urine production. Advise patients to report signs or symptoms of glomerulonephritis to their physician immediately [see Warnings and Precautions (5.5)].
- •
- There may be an increased risk of Myelodysplastic Syndrome and/or Acute Myeloid Leukemia in patients with congenital neutropenia who receive filgrastim products and in patients with breast and lung cancer who receive filgrastim products in conjunction with chemotherapy and/or radiation therapy. Symptoms of MDS and AML may include tiredness, fever, and easy bruising or bleeding. Advise patients to report to their physician signs and symptoms of MDS/AML [see Warnings and Precautions (5.8)].
- •
- Cutaneous vasculitis may occur, which may be signaled by purpura or erythema. Advise patients to report signs or symptoms of vasculitis to their physician immediately [see Warnings and Precautions (5.11)].
- •
- Aortitis may occur. Symptoms may include fever, abdominal pain, malaise, back pain, and increased inflammatory markers. Advise patients to report signs and symptoms of aortitis to their physician immediately [see Warnings and Precautions (5.15)].
Instruct patients who self-administer NIVESTYM using the prefilled syringe or single-dose vial of the:
- •
- Importance of following the applicable Instructions for Use.
- •
- Dangers of reusing needles, syringes, or unused portions of single-dose vials.
- •
- Importance of following local requirements for proper disposal of used syringes, needles, and unused vials.
- •
- Importance of informing the healthcare provider if difficulty occurs when measuring or administering partial contents of the NIVESTYM prefilled syringe. If difficulty occurs, use of the NIVESTYM vial may be considered.
- •
- Difference in product concentration of the NIVESTYM prefilled syringe in comparison to the NIVESTYM vial. When switching patients from the NIVESTYM prefilled syringe to the NIVESTYM vial, or vice versa, ensure that patients understand the correct volume to be administered since the concentration of NIVESTYM differs between the prefilled syringe and the vial.
Patient Information | |
What is NIVESTYM? NIVESTYM is a man-made form of granulocyte colony-stimulating factor (G-CSF). G-CSF is a substance produced by the body. It stimulates the growth of neutrophils, a type of white blood cell important in the body's fight against infection. | |
Do not take NIVESTYM if you have had a serious allergic reaction to human G-CSFs such as filgrastim products or pegfilgrastim products. | |
Before you take NIVESTYM, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | |
How will I receive NIVESTYM?
| |
What are the possible side effects of NIVESTYM? NIVESTYM may cause serious side effects, including:
The most common side effects experienced in patients receiving NIVESTYM include:
These are not all the possible side effects of NIVESTYM. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
How should I store NIVESTYM?
Keep NIVESTYM out of the reach of children. | |
General information about the safe and effective use of NIVESTYM. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NIVESTYM for a condition for which it was not prescribed. Do not give NIVESTYM to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about NIVESTYM that is written for healthcare professionals. | |
What are the ingredients in NIVESTYM? Active ingredient: (filgrastim-aafi) Inactive ingredients: acetate, polysorbate 80, sodium, sorbitol, and water for Injection | |
Manufactured by Hospira, Inc., a Pfizer Company, Lake Forest, IL 60045 USA Distributed by Pfizer Labs, division of Pfizer Inc., New York, NY 10001 USA LAB-0935-5.0 |  |
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: March 2023
Instructions for Use
NIVESTYM (Neye-ves-tim)
(filgrastim-aafi)
injection
Single-Dose Prefilled Syringe
Important
Read the Patient Information for important information you need to know about NIVESTYM before using this Instructions for Use.
Before you use a NIVESTYM prefilled syringe, read this important information.
Storing your prefilled syringe |
- •
- Store the NIVESTYM prefilled syringe in the refrigerator between 36˚F to 46˚F (2˚C to 8˚C).
- •
- Do not freeze.
- •
- Keep the NIVESTYM prefilled syringe in the original carton to protect from light or physical damage.
- •
- Take the prefilled syringe out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- •
- The NIVESTYM prefilled syringe may be allowed to reach room temperature for up to 24 hours. Throw away (dispose of) any NIVESTYM prefilled syringe that has been left at room temperature for longer than 24 hours.
- •
- After you inject your dose, throw away (dispose of) any unused NIVESTYM left in the prefilled syringe. Do not save unused NIVESTYM in the prefilled syringe for later use.
- •
- Keep NIVESTYM and all medicines out of the reach of children.
Using your prefilled syringe |
- •
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
- •
- You should not inject a dose of NIVESTYM less than 0.3 mL (180 mcg) from a NIVESTYM prefilled syringe. A dose less than 0.3 mL cannot be accurately measured using the NIVESTYM prefilled syringe.
- •
- Make sure the name NIVESTYM appears on the carton and prefilled syringe label.
- •
- Do not use a NIVESTYM prefilled syringe after the expiration date on the label.
- •
- Do not shake the NIVESTYM prefilled syringe.
- •
- The prefilled syringe has a needle guard that needs to be activated to cover the needle after the injection is given. The needle guard will help prevent needle stick injuries to anyone who handles the prefilled syringe.
- •
- Do not remove the needle cover from the prefilled syringe until you are ready to inject.
- •
- Do not use the NIVESTYM prefilled syringe if the needle cover is missing.
- •
- Do not use the prefilled syringe if the carton is open or damaged.
- •
- Do not use a prefilled syringe if it has been dropped on a hard surface. The prefilled syringe may be broken even if you cannot see the break. Use a new prefilled syringe.
Call your healthcare provider if you have any questions.
About the NIVESTYM prefilled syringe | |
NIVESTYM prefilled syringe parts (see Figure A). NIVESTYM 300 mcg/0.5 mL prefilled syringe is shown as an example. | |
Figure A | |
What you need for your injection | |
Included in the carton:
Not included in the carton (see Figure B)
| |
Figure B | |
Preparing the NIVESTYM prefilled syringe | |
Step 1: | Find a clean, well-lit flat work surface. |
Step 2: | Take the carton containing the NIVESTYM prefilled syringe out of the refrigerator and leave it unopened on your work surface for at least 30 minutes so that it reaches room temperature. Put the original carton with any unused prefilled syringes back in the refrigerator.
|
Step 3: | Wash your hands with soap and water. |
Step 4: | Remove the prefilled syringe from the carton by the needle guard only. Do not remove the prefilled syringe by its plunger or needle cover. See Figure C. Check to make sure that the needle guard is covering the barrel of the prefilled syringe. |
Figure C | |
Do not push the needle guard over the needle cover before the injection. This may activate or lock the needle guard. See Figure D that shows a needle guard that has not yet been activated. This is how the prefilled syringe looks before use. | |
Figure D | |
If the needle guard is covering the needle that means it has been activated. See Figure E that shows a needle guard that has been activated. This is how the prefilled syringe looks after use. Do not use the NIVESTYM prefilled syringe. Get another prefilled syringe that has not been activated and is ready to use. | |
Figure E | |
Step 5: | Check the expiration date on the NIVESTYM prefilled syringe. Do not use the NIVESTYM prefilled syringe if the expiration date has passed. |
Step 6: | Inspect the medicine and prefilled syringe. Turn the prefilled syringe so you can see the medicine and markings in the window. Make sure that you look at the medicine only through the viewing window on the prefilled syringe (see Figure F). Do not inspect the medicine through the plastic of the needle guard. Make sure the medicine in the prefilled syringe is clear and colorless. |
Figure F | |
| |
Step 7: | Choose the injection site
|
Figure G | |
| |
Step 8: | Clean your injection site with an alcohol wipe. See Figure H.
|
Figure H | |
Step 9: | Hold the prefilled syringe by the needle guard with the needle cover pointing up. Carefully pull the needle cover straight off and away from your body. Throw away the needle cover. Do not recap the needle. See Figure I. |
Figure I | |
Your healthcare provider has prescribed either a "full" syringe dose or a "partial" syringe dose.
| |
Partial dosing | |
Step 10: | Point the needle up and tap gently until the air rises to the top. See Figure J. |
Figure J | |
Step 11: | Holding the prefilled syringe as shown, slowly push up on the plunger rod to push out the extra air and medicine until the end of the conical base (edge) of the plunger stopper lines up with the syringe marking for your prescribed dose. See Figure K for an example of a dose of 0.3 mL. Your dose may be different than the example shown. |
Figure K | |
Giving the NIVESTYM prefilled syringe injection | |
Step 12: | With one hand, gently pinch a fold of skin at the injection site. Hold the pinch. See Figure L. |
Figure L | |
Step 13: | With your other hand, hold the prefilled syringe like you would hold a pencil. Use a quick "dart-like" motion to insert the needle at a 45 to 90 degree angle into the skin as shown. See Figure M. |
Figure M | |
Step 14: | Using slow and constant pressure, press down on the plunger rod until it reaches the bottom. See Figure N. |
Figure N | |
Step 15: | Keep the plunger rod fully pressed down while you carefully pull the needle straight out from the injection site. See Figure O. |
Figure O | |
Step 16: | As you let go of the plunger rod, the needle guard will automatically slide over the needle until the needle is completely covered and the needle guard locks into place. Do not recap the needle. See Figure P. |
Figure P | |
Step 17: | There may be a small amount of blood at the injection site. You can press a cotton ball or gauze over the injection site and hold it for 10 seconds. Do not rub the injection site. You may cover the injection site with a small adhesive bandage, if needed. See Figure Q. |
Figure Q | |
Step 18: | Throw away (dispose of) the syringe as instructed by your healthcare provider or by following the instructions below. See Figure R. |
Figure R | |
Disposing of (throw away) used NIVESTYM prefilled syringes | |
- •
- Put the used prefilled syringe in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) prefilled syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Hospira, Inc.,
a Pfizer Company
Lake Forest, IL 60045 USA
US License No. 1974
Distributed by Pfizer Labs,
division of Pfizer Inc.,
New York, NY 10001 USA
LAB-0938-6.0
For more information go to www.pfizer.com or call 1-800-438-1985.
Revised: February 2024
Instructions for Use
NIVESTYM (Neye-ves-tim)
(filgrastim-aafi)
injection
Single-Dose Vial
Important
Read the Patient Information for important information you need to know about NIVESTYM before using these Instructions for Use.
Before you use a NIVESTYM vial, read this important information:
Storing your NIVESTYM vial
- •
- Store the vial in the refrigerator between 36˚F to 46˚F (2˚C to 8˚C).
- •
- Do not freeze.
- •
- Keep the vial in the original carton to protect from light or physical damage.
- •
- Take the vial out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- •
- Throw away (dispose of) any vial that has been left at room temperature for longer than 24 hours.
- •
- After you inject your dose, throw away (dispose of) any unused NIVESTYM left in the vial. Do not save unused NIVESTYM in the vial for later use.
Keep NIVESTYM and all medicines out of the reach of children.
Using your vial
- •
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
- •
- Make sure the name NIVESTYM appears on the carton and vial label.
- •
- Only use the vial 1 time. Discard (throw away) the vial with any remaining NIVESTYM liquid.
- •
- Do not use a vial after the expiration date on the label.
- •
- Do not shake the vial.
- •
- Do not use the vial if the medicine is cloudy or discolored or contains flakes or particles.
Call your healthcare provider if you have any questions.
Step 1: Prepare
- A.
- Remove the vial from the refrigerator.
Find a clean, well-lit, flat work surface. Place the vial on your clean work surface for 30 minutes and allow it to reach room temperature before you give an injection.- •
- Do not try to warm the vial by using a heat source such as hot water or microwave.
- •
- Do not leave the vial in direct sunlight.
- •
- Do not shake the vial.
- •
- Use the vial only 1 time.
- B.
- Inspect the vial.
Make sure the medicine in the vial is clear and colorless.- •
- Do not use the vial if:
- o
- The medicine is cloudy or discolored or contains flakes or particles.
- o
- The expiration date printed on the label has passed.
- •
- In all cases, use a new vial and call your healthcare provider.
- C.
- Gather all materials needed for your injection.
Wash your hands thoroughly with soap and water. On your clean, well-lit, flat work surface, place:- •
- 1 Vial
- •
- 1 Disposable syringe and needle
- •
- 2 Alcohol wipes
- •
- 1 Cotton ball or gauze pad
- •
- 1 Adhesive bandage
- •
- Sharps disposal container
- •
- Only use the disposable syringes and needles that your healthcare provider prescribes.
- •
- Only use the syringes and needles 1 time. Throw away (dispose of) any used syringes and needles. See Step 5 Finish, for instructions about how to properly dispose of used syringes and needles.
- •
- You should only use a syringe that is marked in tenths of milliliters (mL).
- •
- Your healthcare provider will show you how to measure the correct dose of NIVESTYM. This dose will be measured in milliliters (mL).
Step 2: Get Ready
- D.
- Take the cap off the vial. Clean the rubber stopper with 1 alcohol wipe.
- E.
- Check the carton containing the needle and syringe. If the carton has been opened or damaged, do not use that needle and syringe. Dispose of (throw away) that needle and syringe in the sharps disposal container.
- F.
- Hold the syringe by the barrel with the needle cap pointing up. Carefully pull the needle cap straight off and away from your body.
Pull back on the plunger and draw air into the syringe that is the same amount (mL) as the dose of NIVESTYM that your healthcare provider prescribed.
Important: Throw away the needle cap into the sharps disposal container. Do not recap the needle.- G.
- Keep the vial on the flat work surface and insert the needle straight down through the rubber stopper. Do not insert the needle through the rubber stopper more than 1 time.
- H.
- Push the plunger down and inject all the air from the syringe into the vial of NIVESTYM.
- I.
- Keep the needle in the vial and turn the vial upside down. Make sure that the NIVESTYM liquid is covering the tip of the needle.
- J.
- Keep the vial upside down and slowly pull back on the plunger to fill the syringe barrel with NIVESTYM to the correct marking amount (mL) of medicine that matches the dose your healthcare provider prescribed.
- K.
- Keep the needle in the vial and check for air bubbles in the syringe. If there are air bubbles, gently tap the syringe barrel with your finger until the air bubbles rise to the top. Slowly push the plunger up to push the air bubbles out of the syringe.
- L.
- Keep the tip of the needle in the liquid and again pull the plunger back to the number on the syringe barrel that matches your dose. Check again for air bubbles. The air in the syringe will not hurt you, but too large an air bubble can reduce your dose of NIVESTYM. If there are still air bubbles, repeat the steps above to remove them.
- M.
- Check again to make sure that you have the correct dose in the syringe. It is important that you use the exact dose prescribed by your healthcare provider. Do not remove the needle from the vial. Lay the vial down on its side with the needle still in the vial.
Step 3: Select and Prepare the Injection Site
- N.
- Prepare and clean your injection site.
You can use:
- •
- Thigh
- •
- Stomach area (abdomen), except for a 2-inch area right around your navel (belly button)
- •
- Upper outer area of your buttocks (only if someone else is giving you the injection)
- •
- Outer area of upper arm (only if someone else is giving you the injection)
Clean your injection site with a clean alcohol wipe.
Let your skin dry. - •
- Do not touch this area again before injecting.
- •
- If you want to use the same injection site, make sure it is not the same spot on the injection site area you used for a previous injection.
- •
- Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks.
Step 4: Subcutaneous (under the skin) injection
- O.
- Remove the prepared syringe and needle from the vial.
- P.
- Pinch your injection site to create a firm surface.
Important: Keep skin pinched while injecting. - Q.
- Hold the pinch. Insert the needle into the skin at a 45 to 90 degree angle.
- R.
- Using slow and constant pressure, push the plunger until it reaches the bottom.
When done gently pull the needle out of the injection site at the same 45 to 90 degree angle used to insert it.
- S.
- Dispose of (throw away) the used needle and syringe.
- •
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles, and syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- T.
- Examine the injection site.
If there is blood, press a cotton ball or gauze pad on your injection site. Do not rub the injection site. Apply an adhesive bandage if needed.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Hospira, Inc.,
a Pfizer Company
Lake Forest, IL 60045 USA
US License No. 1974
Distributed by Pfizer Labs,
division of Pfizer Inc.,
New York, NY 10001 USA
LAB-0937-5.0
For more information go to www.pfizer.com or call 1-800-438-1985.
Revised: August 2023
Highlights
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NIVESTYM safely and effectively. See full prescribing information for NIVESTYM. NIVESTYM™ (filgrastim-aafi) injection, for subcutaneous or intravenous use Initial U.S. Approval: 2018 NIVESTYM (filgrastim-aafi) is biosimilar* to NEUPOGEN (filgrastim). INDICATIONS AND USAGENIVESTYM is a leukocyte growth factor indicated to
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSCONTRAINDICATIONSPatients with a history of serious allergic reactions to human granulocyte colony-stimulating factors such as filgrastim products or pegfilgrastim products. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions in patients:
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2024 |
Indications and Usage
1 INDICATIONS AND USAGE
1.1 Patients with Cancer Receiving Myelosuppressive Chemotherapy
NIVESTYM is indicated to decrease the incidence of infection‚ as manifested by febrile neutropenia‚ in patients with nonmyeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a significant incidence of severe neutropenia with fever [see Clinical Studies (14.1)].
1.2 Patients with Acute Myeloid Leukemia Receiving Induction or Consolidation Chemotherapy
NIVESTYM is indicated for reducing the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy treatment of patients with acute myeloid leukemia (AML) [see Clinical Studies (14.2)].
1.3 Patients with Cancer Undergoing Bone Marrow Transplantation
NIVESTYM is indicated to reduce the duration of neutropenia and neutropenia-related clinical sequelae‚ e.g.‚ febrile neutropenia, in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplantation [see Clinical Studies (14.3)].
1.4 Patients Undergoing Autologous Peripheral Blood Progenitor Cell Collection and Therapy
NIVESTYM is indicated for the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis [see Clinical Studies (14.4)].
1.5 Patients with Severe Chronic Neutropenia
NIVESTYM is indicated for chronic administration to reduce the incidence and duration of sequelae of neutropenia (e.g.‚ fever‚ infections‚ oropharyngeal ulcers) in symptomatic patients with congenital neutropenia‚ cyclic neutropenia‚ or idiopathic neutropenia [see Clinical Studies (14.5)].
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Patients with Cancer Receiving Myelosuppressive Chemotherapy or Induction and/or Consolidation Chemotherapy for AML
The recommended starting dosage of NIVESTYM is 5 mcg/kg/day‚ administered as a single daily injection by subcutaneous injection‚ by short intravenous infusion (15 to 30 minutes)‚ or by continuous intravenous infusion. Obtain a complete blood count (CBC) and platelet count before instituting NIVESTYM therapy and monitor twice weekly during therapy. Consider dose escalation in increments of 5 mcg/kg for each chemotherapy cycle‚ according to the duration and severity of the absolute neutrophil count (ANC) nadir. Recommend stopping NIVESTYM if the ANC increases beyond 10‚000/mm3 [see Warnings and Precautions (5.10)].
Administer NIVESTYM at least 24 hours after cytotoxic chemotherapy. Do not administer NIVESTYM within the 24-hour period prior to chemotherapy [see Warnings and Precautions (5.13)]. A transient increase in neutrophil count is typically seen 1 to 2 days after initiation of NIVESTYM therapy. Therefore, to ensure a sustained therapeutic response‚ administer NIVESTYM daily for up to 2 weeks or until the ANC has reached 10‚000/mm3 following the expected chemotherapy-induced neutrophil nadir. The duration of NIVESTYM therapy needed to attenuate chemotherapy-induced neutropenia may be dependent on the myelosuppressive potential of the chemotherapy regimen employed.
2.2 Dosage in Patients with Cancer Undergoing Bone Marrow Transplantation
The recommended dosage of NIVESTYM following bone marrow transplantation (BMT) is 10 mcg/kg/day given as an intravenous infusion no longer than 24 hours. Administer the first dose of NIVESTYM at least 24 hours after cytotoxic chemotherapy and at least 24 hours after bone marrow infusion. Monitor CBCs and platelet counts frequently following marrow transplantation.
During the period of neutrophil recovery‚ titrate the daily dosage of NIVESTYM against the neutrophil response (see Table 1).
| Absolute Neutrophil Count | NIVESTYM Dosage Adjustment |
|---|---|
| |
When ANC greater than 1,000/mm3 for 3 consecutive days | Reduce to 5 mcg/kg/day* |
Then, if ANC remains greater than 1,000/mm3 for 3 more consecutive days | Discontinue NIVESTYM |
Then, if ANC decreases to less than 1,000/mm3 | Resume at 5 mcg/kg/day |
2.3 Dosage in Patients Undergoing Autologous Peripheral Blood Progenitor Cell Collection and Therapy
The recommended dosage of NIVESTYM for the mobilization of autologous peripheral blood progenitor cells (PBPC) is 10 mcg/kg/day given by subcutaneous injection. Administer NIVESTYM for at least 4 days before the first leukapheresis procedure and continue until the last leukapheresis. Although the optimal duration of NIVESTYM administration and leukapheresis schedule have not been established‚ administration of filgrastim for 6 to 7 days with leukaphereses on days 5‚ 6‚ and 7 was found to be safe and effective [see Clinical Studies (14.4)]. Monitor neutrophil counts after 4 days of NIVESTYM‚ and discontinue NIVESTYM if the white blood cell (WBC) count rises to greater than 100‚000/mm3.
2.4 Dosage in Patients with Severe Chronic Neutropenia
Prior to starting NIVESTYM in patients with suspected chronic neutropenia, confirm the diagnosis of severe chronic neutropenia (SCN) by evaluating serial CBCs with differential and platelet counts‚ and evaluating bone marrow morphology and karyotype. The use of NIVESTYM prior to confirmation of a correct diagnosis of SCN may impair diagnostic efforts and may thus impair or delay evaluation and treatment of an underlying condition‚ other than SCN‚ causing the neutropenia.
The recommended starting dosage in patients with Congenital Neutropenia is 6 mcg/kg as a twice daily subcutaneous injection and the recommended starting dosage in patients with Idiopathic or Cyclic Neutropenia is 5 mcg/kg as a single daily subcutaneous injection.
Dosage Adjustments in Patients with Severe Chronic Neutropenia
Chronic daily administration is required to maintain clinical benefit. Individualize the dosage based on the patient's clinical course as well as ANC. In the SCN postmarketing surveillance study, the reported median daily doses of filgrastim were: 6 mcg/kg (congenital neutropenia), 2.1 mcg/kg (cyclic neutropenia), and 1.2 mcg/kg (idiopathic neutropenia). In rare instances, patients with congenital neutropenia have required doses of filgrastim greater than or equal to 100 mcg/kg/day.
Monitor CBCs for Dosage Adjustments
During the initial 4 weeks of NIVESTYM therapy and during the 2 weeks following any dosage adjustment‚ monitor CBCs with differential and platelet counts. Once a patient is clinically stable‚ monitor CBCs with differential and platelet counts monthly during the first year of treatment. Thereafter, if the patient is clinically stable, less frequent routine monitoring is recommended.
2.5 Important Administration Instructions
Patient self-administration and administration by a caregiver may benefit from training by a healthcare professional. Training should aim to demonstrate to those patients and caregivers how to measure the dose using the prefilled syringe, and the focus should be on ensuring that a patient or caregiver can successfully perform all of the steps in the Instructions for Use of NIVESTYM prefilled syringe with BD UltraSafe Plus™ Passive Needle Guard. If a patient or caregiver is not able to demonstrate that they can measure the dose and administer the product successfully, you should consider whether the patient is an appropriate candidate for self-administration of NIVESTYM [see Instructions for Use].
NIVESTYM prefilled syringe with BD UltraSafe Plus™ Passive Needle Guard is not designed to allow for direct administration of doses of less than 0.3 mL (180 mcg). The spring-mechanism of the needle guard apparatus affixed to the prefilled syringe interferes with the visibility of the graduation markings on the syringe barrel corresponding to 0.1 mL and 0.2 mL. The visibility of these markings is necessary to accurately measure doses of NIVESTYM less than 0.3 mL (180 mcg) for direct administration. Thus, the direct administration to patients requiring doses of less than 0.3 mL (180 mcg) is not recommended due to the potential for dosing errors. For direct administration of doses less than 0.3 mL (180 mcg) use NIVESTYM single-dose vial.
NIVESTYM is supplied in single-dose vials (for subcutaneous use or intravenous infusion) and single-dose prefilled syringes (for subcutaneous use) [see Dosage Forms and Strengths (3)]. Prior to use‚ remove the vial or prefilled syringe from the refrigerator and allow NIVESTYM to reach room temperature for a minimum of 30 minutes. If not used immediately, the vial or prefilled syringe may be stored at room temperature [between 20°C to 25°C (68°F to 77°F)] for up to 24 hours. Discard any vial or prefilled syringe left at room temperature for greater than 24 hours. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit (the solution is clear and colorless). Do not administer NIVESTYM if particulates or discoloration are observed.
Discard unused portion of NIVESTYM in vials or prefilled syringes; do not re-enter the vial. Do not save unused drug for later administration.
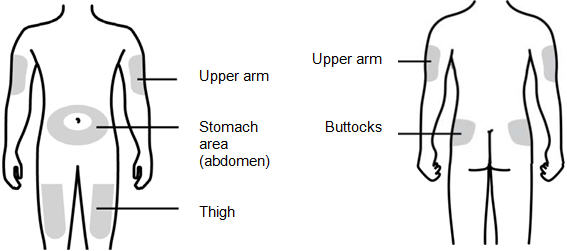
Subcutaneous Injection
Inject NIVESTYM subcutaneously in the outer area of upper arms, abdomen, thighs, or upper outer areas of the buttock. If patients or caregivers are to administer NIVESTYM, instruct them in appropriate injection technique and ask them to follow the subcutaneous injection procedures in the Instructions for Use for the vial or prefilled syringe [see Patient Counseling Information (17)].
Training by the healthcare provider should aim to demonstrate to those patients and caregivers how to measure the dose of NIVESTYM, and the focus should be on ensuring that a patient or caregiver can successfully perform all of the steps in the Instructions for Use for the vial or prefilled syringe. If a patient or caregiver is not able to demonstrate that they can measure the dose and administer the product successfully, you should consider whether the patient is an appropriate candidate for self-administration of NIVESTYM or whether the patient would benefit from a different NIVESTYM presentation. If a patient or caregiver experiences difficulty measuring the required dose, especially if it is other than the entire contents of the NIVESTYM prefilled syringe, use of the NIVESTYM vial may be considered.
If the patient or caregiver misses a dose of NIVESTYM, instruct them to contact their healthcare provider.
Administration Instructions for the Prefilled Syringe
The NIVESTYM syringe plunger stopper and needle cover are not made with natural rubber latex.
Administration Instructions for Dilution (Vial Only)
If required for intravenous administration‚ NIVESTYM (vial only) may be diluted in 5% Dextrose Injection, USP from a concentration of 300 mcg/mL to 5 mcg/mL (do not dilute to a final concentration less than 5 mcg/mL). NIVESTYM diluted to concentrations from 5 mcg/mL to 15 mcg/mL should be protected from adsorption to plastic materials by the addition of Albumin (Human) to a final concentration of 2 mg/mL. When diluted in 5% Dextrose Injection, USP or 5% Dextrose plus Albumin (Human)‚ NIVESTYM is compatible with glass bottles‚ polyvinyl chloride (PVC) and polyolefin intravenous bags‚ and polypropylene syringes. Do not dilute with saline at any time because the product may precipitate.
Diluted NIVESTYM solution can be stored at room temperature for up to 24 hours. This 24-hour time period includes the time during room temperature storage of the infusion solution and the duration of the infusion.
Dosage Forms and Strengths
Contraindications
4 CONTRAINDICATIONS
NIVESTYM is contraindicated in patients with a history of serious allergic reactions to human granulocyte colony-stimulating factors such as filgrastim products or pegfilgrastim products [see Warnings and Precautions (5.3)].
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Splenic Rupture
Splenic rupture, including fatal cases, has been reported following the administration of filgrastim products. Evaluate patients who report left upper abdominal or shoulder pain for an enlarged spleen or splenic rupture.
5.2 Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome (ARDS) has been reported in patients receiving filgrastim products. Evaluate patients who develop fever and lung infiltrates or respiratory distress for ARDS. Discontinue NIVESTYM in patients with ARDS.
5.3 Serious Allergic Reactions
Serious allergic reactions, including anaphylaxis, have been reported in patients receiving filgrastim products. The majority of reported events occurred upon initial exposure. Provide symptomatic treatment for allergic reactions. Allergic reactions, including anaphylaxis, in patients receiving filgrastim products can recur within days after the discontinuation of initial anti-allergic treatment. Permanently discontinue NIVESTYM in patients with serious allergic reactions. NIVESTYM is contraindicated in patients with a history of serious allergic reactions to human granulocyte colony-stimulating factors such as filgrastim products or pegfilgrastim products.
5.4 Sickle Cell Disorders
Severe and sometimes fatal sickle cell crises can occur in patients with sickle cell disorders receiving filgrastim products. Discontinue NIVESTYM if sickle cell crisis occurs.
5.5 Glomerulonephritis
Glomerulonephritis has occurred in patients receiving filgrastim products. The diagnoses were based upon azotemia, hematuria (microscopic and macroscopic), proteinuria, and renal biopsy. Generally, events of glomerulonephritis resolved after dose reduction or discontinuation of filgrastim products. If glomerulonephritis is suspected, evaluate for cause. If causality is likely, consider dose-reduction or interruption of NIVESTYM.
5.6 Alveolar Hemorrhage and Hemoptysis
Alveolar hemorrhage manifesting as pulmonary infiltrates and hemoptysis requiring hospitalization have been reported in healthy donors treated with filgrastim products undergoing peripheral blood progenitor cell (PBPC) collection mobilization. Hemoptysis resolved with discontinuation of filgrastim products. The use of NIVESTYM for PBPC mobilization in healthy donors is not an approved indication.
5.7 Capillary Leak Syndrome
Capillary leak syndrome (CLS) has been reported after G-CSF administration, including filgrastim products, and is characterized by hypotension, hypoalbuminemia, edema and hemoconcentration. Episodes vary in frequency, severity and may be life-threatening if treatment is delayed. Patients who develop symptoms of capillary leak syndrome should be closely monitored and receive standard symptomatic treatment, which may include a need for intensive care.
5.8 Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML)
Patients with Severe Chronic Neutropenia
Confirm the diagnosis of SCN before initiating NIVESTYM therapy.
MDS and AML have been reported to occur in the natural history of congenital neutropenia without cytokine therapy. Cytogenetic abnormalities, transformation to MDS, and AML have also been observed in patients treated with filgrastim products for SCN. Based on available data including a postmarketing surveillance study, the risk of developing MDS and AML appears to be confined to the subset of patients with congenital neutropenia. Abnormal cytogenetics and MDS have been associated with the eventual development of myeloid leukemia. The effect of filgrastim products on the development of abnormal cytogenetics and the effect of continued filgrastim products administration in patients with abnormal cytogenetics or MDS are unknown. Monitor patients for signs and symptoms of MDS/AML in these settings. If a patient with SCN develops abnormal cytogenetics or myelodysplasia‚ the risks and benefits of continuing NIVESTYM should be carefully considered.
5.9 Thrombocytopenia
Thrombocytopenia has been reported in patients receiving filgrastim products. Monitor platelet counts.
5.10 Leukocytosis
Patients with Cancer Receiving Myelosuppressive Chemotherapy
White blood cell counts of 100‚000/mm3 or greater were observed in approximately 2% of patients receiving filgrastim at dosages above 5 mcg/kg/day. In patients with cancer receiving NIVESTYM as an adjunct to myelosuppressive chemotherapy‚ to avoid the potential risks of excessive leukocytosis‚ it is recommended that NIVESTYM therapy be discontinued if the ANC surpasses 10‚000/mm3 after the chemotherapy-induced ANC nadir has occurred. Monitor CBCs at least twice weekly during therapy. Dosages of NIVESTYM that increase the ANC beyond 10‚000/mm3 may not result in any additional clinical benefit. In patients with cancer receiving myelosuppressive chemotherapy‚ discontinuation of filgrastim therapy usually resulted in a 50% decrease in circulating neutrophils within 1 to 2 days‚ with a return to pretreatment levels in 1 to 7 days.
5.11 Cutaneous Vasculitis
Cutaneous vasculitis has been reported in patients treated with filgrastim products. In most cases‚ the severity of cutaneous vasculitis was moderate or severe. Most of the reports involved patients with SCN receiving long-term filgrastim therapy. Hold NIVESTYM therapy in patients with cutaneous vasculitis. NIVESTYM may be started at a reduced dose when the symptoms resolve and the ANC has decreased.
5.12 Potential Effect on Malignant Cells
NIVESTYM is a growth factor that primarily stimulates neutrophils. The granulocyte colony-stimulating factor (G-CSF) receptor through which NIVESTYM acts has also been found on tumor cell lines. The possibility that NIVESTYM acts as a growth factor for any tumor type cannot be excluded. The safety of filgrastim products in chronic myeloid leukemia (CML) and myelodysplasia has not been established.
When NIVESTYM is used to mobilize PBPC‚ tumor cells may be released from the marrow and subsequently collected in the leukapheresis product. The effect of reinfusion of tumor cells has not been well studied‚ and the limited data available are inconclusive.
5.13 Simultaneous Use with Chemotherapy and Radiation Therapy Not Recommended
The safety and efficacy of NIVESTYM given simultaneously with cytotoxic chemotherapy have not been established. Because of the potential sensitivity of rapidly dividing myeloid cells to cytotoxic chemotherapy‚ do not use NIVESTYM in the period 24 hours before through 24 hours after the administration of cytotoxic chemotherapy [see Dosage and Administration (2.2)].
The safety and efficacy of NIVESTYM have not been evaluated in patients receiving concurrent radiation therapy. Avoid the simultaneous use of NIVESTYM with chemotherapy and radiation therapy.
5.14 Nuclear Imaging
Increased hematopoietic activity of the bone marrow in response to growth factor therapy has been associated with transient positive bone-imaging changes. This should be considered when interpreting bone-imaging results.
5.15 Aortitis
Aortitis has been reported in patients receiving filgrastim products. It may occur as early as the first week after start of therapy. Manifestations may include generalized signs and symptoms such as fever, abdominal pain, malaise, back pain, and increased inflammatory markers (e.g., c-reactive protein and white blood cell count). Consider aortitis in patients who develop these signs and symptoms without known etiology. Discontinue NIVESTYM if aortitis is suspected.
Adverse Reactions
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- Splenic Rupture [see Warnings and Precautions (5.1)]
- •
- Acute Respiratory Distress Syndrome [see Warnings and Precautions (5.2)]
- •
- Serious Allergic Reactions [see Warnings and Precautions (5.3)]
- •
- Sickle Cell Disorders [see Warnings and Precautions (5.4)]
- •
- Glomerulonephritis [see Warnings and Precautions (5.5)]
- •
- Alveolar Hemorrhage and Hemoptysis [see Warnings and Precautions (5.6)]
- •
- Capillary Leak Syndrome [see Warnings and Precautions (5.7)]
- •
- Myelodysplastic Syndrome [see Warnings and Precautions (5.8)]
- •
- Acute Myeloid Leukemia [see Warnings and Precautions (5.8)]
- •
- Thrombocytopenia [see Warnings and Precautions (5.9)]
- •
- Leukocytosis [see Warnings and Precautions (5.10)]
- •
- Cutaneous Vasculitis [see Warnings and Precautions (5.11)]
- •
- Aortitis [see Warnings and Precautions (5.15)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions in Patients with Cancer Receiving Myelosuppressive Chemotherapy
The following adverse reaction data in Table 2 are from three randomized, placebo-controlled studies in patients with:
- •
- small cell lung cancer receiving standard dose chemotherapy with cyclophosphamide‚ doxorubicin‚ and etoposide (Study 1)
- •
- small cell lung cancer receiving ifosfamide, doxorubicin‚ and etoposide (Study 2), and
- •
- non-Hodgkin's lymphoma (NHL) receiving doxorubicin, cyclophosphamide, vindesine, bleomycin, methylprednisolone, and methotrexate ("ACVBP") or mitoxantrone, ifosfamide, mitoguazone, teniposide, methotrexate, folinic acid, methylprednisolone, and methotrexate ("VIM3") (Study 3).
A total of 451 patients were randomized to receive subcutaneous filgrastim 230 mcg/m2 (Study 1), 240 mcg/m2 (Study 2) or 4 or 5 mcg/kg/day (Study 3) (n = 294) or placebo (n = 157). The patients in these studies were median age 61 (range 29 to 78) years and 64% were male. The ethnicity was 95% Caucasian, 4% African American, and 1% Asian.
| System Organ Class Preferred Term | Filgrastim (N = 294) | Placebo (N = 157) |
|---|---|---|
| ||
Blood and lymphatic system disorders | ||
Thrombocytopenia | 38% | 29% |
Gastrointestinal disorders | ||
Nausea | 43% | 32% |
General disorders and administration site conditions | ||
Pyrexia | 48% | 29% |
Chest pain | 13% | 6% |
Pain | 12% | 6% |
Fatigue | 20% | 10% |
Musculoskeletal and connective tissue disorders | ||
Back pain | 15% | 8% |
Arthralgia | 9% | 2% |
Bone pain | 11% | 6% |
Pain in extremity* | 7% | 3% |
Nervous system disorders | ||
Dizziness | 14% | 3% |
Respiratory, thoracic and mediastinal disorders | ||
Cough | 14% | 8% |
Dyspnea | 13% | 8% |
Skin and subcutaneous tissue disorders | ||
Rash | 14% | 5% |
Investigations | ||
Blood lactate dehydrogenase increased | 6% | 1% |
Blood alkaline phosphatase increased | 6% | 1% |
Adverse events with ≥ 5% higher incidence in filgrastim patients compared to placebo and associated with the sequelae of the underlying malignancy or cytotoxic chemotherapy delivered included anemia, constipation, diarrhea, oral pain, vomiting, asthenia, malaise, edema peripheral, hemoglobin decreased, decreased appetite, oropharyngeal pain, and alopecia.
Adverse Reactions in Patients with Acute Myeloid Leukemia
Adverse reaction data below are from a randomized, double-blind, placebo-controlled study in patients with AML (Study 4) who received an induction chemotherapy regimen of intravenous daunorubicin days 1, 2, and 3; cytosine arabinoside days 1 to 7; and etoposide days 1 to 5 and up to 3 additional courses of therapy (induction 2, and consolidation 1, 2) of intravenous daunorubicin, cytosine arabinoside, and etoposide. The safety population included 518 patients randomized to receive either 5 mcg/kg/day filgrastim (n = 257) or placebo (n = 261). The median age was 54 (range 16 to 89) years and 54% were male.
Adverse reactions with ≥ 2% higher incidence in filgrastim patients compared to placebo included epistaxis, back pain, pain in extremity, erythema, and rash maculo-papular.
Adverse events with ≥ 2% higher incidence in filgrastim patients compared to placebo and associated with the sequelae of the underlying malignancy or cytotoxic chemotherapy included diarrhea, constipation, and transfusion reaction.
Adverse Reactions in Patients with Cancer Undergoing Bone Marrow Transplantation
The following adverse reaction data are from one randomized, no treatment-controlled study in patients with acute lymphoblastic leukemia or lymphoblastic lymphoma receiving high-dose chemotherapy (cyclophosphamide or cytarabine, and melphalan) and total body irradiation (Study 5) and one randomized, no treatment-controlled study in patients with Hodgkin's disease (HD) and NHL undergoing high-dose chemotherapy and autologous bone marrow transplantation (Study 6). Patients receiving autologous bone marrow transplantation only were included in the analysis. A total of 100 patients received either 30 mcg/kg/day as a 4-hour infusion (Study 5) or 10 mcg/kg/day or 30 mcg/kg/day as a 24-hour infusion (Study 6) filgrastim (n = 72), no treatment control or placebo (n = 28). The median age was 30 (range 15 to 57) years, 57% were male.
Adverse reactions with ≥ 5% higher incidence in filgrastim patients compared to patients receiving no filgrastim included rash and hypersensitivity.
Adverse reactions in patients receiving intensive chemotherapy followed by autologous BMT with ≥ 5% higher incidence in filgrastim patients compared to patients receiving no filgrastim included thrombocytopenia, anemia, hypertension, sepsis, bronchitis, and insomnia.
Adverse Reactions in Patients with Cancer Undergoing Autologous Peripheral Blood Progenitor Cell Collection
The adverse reaction data in Table 3 are from a series of 7 trials in patients with cancer undergoing mobilization of autologous peripheral blood progenitor cells for collection by leukapheresis. Patients (n = 166) in all these trials underwent a similar mobilization/collection regimen: filgrastim was administered for 6 to 8 days‚ in most cases the apheresis procedure occurred on days 5‚ 6, and 7. The dosage of filgrastim ranged between 5 to 30 mcg/kg/day and was administered subcutaneously by injection or continuous infusion. The median age was 39 (range 15 to 67) years, and 48% were male.
| System Organ Class Preferred Term | Mobilization Phase (N = 166) |
|---|---|
Musculoskeletal and connective tissue disorders | |
Bone pain | 30% |
General disorders and administration site conditions | |
Pyrexia | 16% |
Investigations | |
Blood alkaline phosphatase increased | 11% |
Nervous system disorders | |
Headache | 10% |
Adverse Reactions in Patients with Severe Chronic Neutropenia
The following adverse reaction data were identified in a randomized, controlled study in patients with SCN receiving filgrastim (Study 7). 123 patients were randomized to a 4-month observation period followed by subcutaneous filgrastim treatment or immediate subcutaneous filgrastim treatment. The median age was 12 years (range 7 months to 76 years) and 46% were male. The dosage of filgrastim was determined by the category of neutropenia. Initial dosage of filgrastim:
- •
- Idiopathic neutropenia: 3.6 mcg/kg/day
- •
- Cyclic neutropenia: 6 mcg/kg/day
- •
- Congenital neutropenia: 6 mcg/kg/day divided 2 times per day
The dosage was increased incrementally to 12 mcg/kg/day divided 2 times per day if there was no response.
Adverse reactions with ≥ 5% higher incidence in filgrastim patients compared to patients receiving no filgrastim included arthralgia, bone pain, back pain, muscle spasms, musculoskeletal pain, pain in extremity, splenomegaly, anemia, upper respiratory tract infection, and urinary tract infection (upper respiratory tract infection and urinary tract infection were higher in the filgrastim arm, total infection related events were lower in filgrastim-treated patients), epistaxis, chest pain, diarrhea, hypoesthesia, and alopecia.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of filgrastim products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- •
- splenic rupture and splenomegaly (enlarged spleen) [see Warnings and Precautions (5.1)]
- •
- acute respiratory distress syndrome [see Warnings and Precautions (5.2)]
- •
- anaphylaxis [see Warnings and Precautions (5.3)]
- •
- sickle cell disorders [see Warnings and Precautions (5.4)]
- •
- glomerulonephritis [see Warnings and Precautions (5.5)]
- •
- alveolar hemorrhage and hemoptysis [see Warnings and Precautions (5.6)]
- •
- capillary leak syndrome [see Warnings and Precautions (5.7)]
- •
- leukocytosis [see Warnings and Precautions (5.10)]
- •
- cutaneous vasculitis [see Warnings and Precautions (5.11)]
- •
- Sweet's syndrome (acute febrile neutrophilic dermatosis)
- •
- decreased bone density and osteoporosis in pediatric patients receiving chronic treatment with filgrastim products.
- •
- myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in patients with breast and lung cancer receiving chemotherapy and/or radiotherapy [see Warnings and Precautions (5.8)]
- •
- aortitis [see Warnings and Precautions (5.15)]
- •
- extramedullary hematopoiesis
Use in Specific Populations
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published studies, including several observational studies of pregnancy outcomes in women exposed to filgrastim products and those who were unexposed, have not established an association with filgrastim products use during pregnancy and major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). Reports in the scientific literature have described transplacental passage of filgrastim in pregnant women when administered ≤ 30 hours prior to preterm delivery (≤ 30 weeks gestation). In animal reproduction studies, effects of filgrastim on prenatal development have been studied in rats and rabbits. No malformations were observed in either species. No maternal or fetal effects were observed in pregnant rats at doses up to 58 times the human doses. Filgrastim has been shown to have adverse effects in pregnant rabbits at doses 2 to 10 times higher than the human doses (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Human data
Several observational studies based on the Severe Chronic Neutropenia International Registry (SCNIR) described pregnancy outcomes in women with severe chronic neutropenia (SCN) who were exposed to filgrastim products during pregnancy and women with SCN who were unexposed. No major differences were seen between treated and untreated women with respect to pregnancy outcome (including miscarriage and preterm labor), newborn complications (including birth weight) and infections. Methodological limitations of these studies, include small sample size, and lack of generalizability due to the underlying maternal condition.
Animal data
Effects of filgrastim on prenatal development have been studied in rats and rabbits. No malformations were observed in either species. Filgrastim has been shown to have adverse effects in pregnant rabbits at doses 2 to 10 times higher than the human doses. In pregnant rabbits showing signs of maternal toxicity, reduced embryo-fetal survival (at 20 and 80 mcg/kg/day) and increased abortions (at 80 mcg/kg/day) were observed. In pregnant rats, no maternal or fetal effects were observed at doses up to 575 mcg/kg/day, which is approximately 58 times higher than the human dose of 10 mcg/kg/day.
Offspring of rats administered filgrastim during the peri-natal and lactation periods exhibited a delay in external differentiation and growth retardation (≥ 20 mcg/kg/day) and slightly reduced survival rate (100 mcg/kg/day).
8.2 Lactation
Risk Summary
There is published literature documenting transfer of filgrastim into human milk. There are a few case reports describing the use of filgrastim in breastfeeding mothers with no adverse effects noted in the infants. There are no data on the effects of filgrastim products on milk production. Other filgrastim products are secreted poorly into breast milk, and filgrastim products are not absorbed orally by neonates. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for NIVESTYM and any potential adverse effects on the breastfed child from NIVESTYM or from the underlying maternal condition.
8.4 Pediatric Use
NIVESTYM prefilled syringe with BD UltraSafe Plus™ Passive Needle Guard may not accurately measure volumes less than 0.3 mL due to the needle spring mechanism design. Therefore, the direct administration of a volume less than 0.3 mL using NIVESTYM prefilled syringe is not recommended due to the potential for dosing errors. For direct administration of doses less than 0.3 mL (180 mcg) use NIVESTYM single-dose vial.
In patients with cancer receiving myelosuppressive chemotherapy‚ 15 pediatric patients median age 2.6 (range 1.2 to 9.4) years with neuroblastoma were treated with myelosuppressive chemotherapy (cyclophosphamide‚ cisplatin‚ doxorubicin‚ and etoposide) followed by subcutaneous filgrastim at doses of 5, 10, or 15 mcg/kg/day for 10 days (n = 5/dose) (Study 8). The pharmacokinetics of filgrastim in pediatric patients after chemotherapy were similar to those in adults receiving the same weight-normalized doses, suggesting no age-related differences in the pharmacokinetics of filgrastim. In this population‚ filgrastim was well-tolerated. There was one report of palpable splenomegaly and one report of hepatosplenomegaly associated with filgrastim therapy; however‚ the only consistently reported adverse event was musculoskeletal pain‚ which is no different from the experience in the adult population.
The safety and effectiveness of NIVESTYM have been established in pediatric patients with SCN [see Clinical Studies (14.5)]. Use of NIVESTYM for this indication is supported by NIVESTYM’s approval as a biosimilar to filgrastim and evidence from a phase 3 study (Study 7) to assess the safety and efficacy of filgrastim in the treatment of SCN where 123 patients with a median age of 12 years (range 7 months to 76 years) were studied. Of the 123 patients, 12 were infants (7 months to 2 years of age), 49 were children (2 to 12 years of age), and 9 were adolescents (12 to 16 years of age). Additional information is available from a SCN postmarketing surveillance study, which includes long-term follow-up of patients in the clinical studies and information from additional patients who entered directly into the postmarketing surveillance study. Of the 731 patients in the surveillance study, 429 were pediatric patients < 18 years of age (range 0.9 to 17) [see Indications and Usage (1.5), Dosage and Administration (2.5), and Clinical Studies (14.5)].
Long-term follow-up data from the postmarketing surveillance study suggest that height and weight are not adversely affected in patients who received up to 5 years of filgrastim treatment. Limited data from patients who were followed in the phase 3 study for 1.5 years did not suggest alterations in sexual maturation or endocrine function.
Pediatric patients with congenital types of neutropenia (Kostmann's syndrome, congenital agranulocytosis, or Schwachman-Diamond syndrome) have developed cytogenetic abnormalities and have undergone transformation to MDS and AML while receiving chronic filgrastim treatment. The relationship of these events to filgrastim product administration is unknown [see Warnings and Precautions (5.8) and Adverse Reactions (6)].
8.5 Geriatric Use
Among 855 subjects enrolled in 3 randomized, placebo-controlled trials of filgrastim-treated patients receiving myelosuppressive chemotherapy, there were 232 subjects age 65 or older, and 22 subjects age 75 or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
Clinical studies of filgrastim in other approved indications (i.e., BMT recipients, PBPC mobilization, and SCN) did not include sufficient numbers of subjects aged 65 and older to determine whether elderly subjects respond differently from younger subjects.
Overdosage
10 OVERDOSAGE
The maximum tolerated dose of filgrastim products has not been determined. In filgrastim clinical trials of patients with cancer receiving myelosuppressive chemotherapy‚ WBC counts > 100‚000/mm3 have been reported in less than 5% of patients‚ but were not associated with any reported adverse clinical effects. Patients in the BMT studies received up to 138 mcg/kg/day without toxic effects‚ although there was a flattening of the dose response curve above daily doses of greater than 10 mcg/kg/day.
Description
11 DESCRIPTION
Filgrastim-aafi is a 175 amino acid human granulocyte colony-stimulating factor (G-CSF) manufactured by recombinant DNA technology. NIVESTYM is produced by Escherichia coli (E coli) bacteria into which has been inserted the human granulocyte colony-stimulating factor gene. NIVESTYM has a molecular weight of 18‚799 daltons. The protein has an amino acid sequence that is identical to the natural sequence predicted from human DNA sequence analysis‚ except for the addition of an N-terminal methionine necessary for expression in E coli. Because NIVESTYM is produced in E coli‚ the product is non-glycosylated and thus differs from G-CSF isolated from a human cell.
NIVESTYM is a sterile‚ clear‚ colorless‚ preservative-free liquid containing filgrastim-aafi injection for subcutaneous or intravenous use. The product is available in single-dose vials for subcutaneous or intravenous use and prefilled syringes for subcutaneous use. The single-dose vials contain either 300 mcg/mL or 480 mcg/1.6 mL of filgrastim-aafi. The single-dose prefilled syringes contain either 300 mcg/0.5 mL or 480 mcg/0.8 mL of filgrastim-aafi. The final pH range of the NIVESTYM drug product solution is 3.8 to 4.3. See Table 4 below for product composition of each single-dose vial or prefilled syringe.
| 300 mcg/mL Vial | 480 mcg/1.6 mL Vial | 300 mcg/0.5 mL Syringe | 480 mcg/0.8 mL Syringe | |
|---|---|---|---|---|
| ||||
Filgrastim-aafi | 300 mcg | 480 mcg | 300 mcg | 480 mcg |
Acetate | 0.59 mg | 0.94 mg | 0.295 mg | 0.472 mg |
Polysorbate 80 | 0.04 mg | 0.064 mg | 0.02 mg | 0.032 mg |
Sodium | 0.035 mg | 0.056 mg | 0.0175 mg | 0.028 mg |
Sorbitol | 50 mg | 80 mg | 25 mg | 40 mg |
Water for Injection USP | 1 mL | 1.6 mL | 0.5 mL | 0.8 mL |
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Colony-stimulating factors are glycoproteins which act on hematopoietic cells by binding to specific cell surface receptors and stimulating proliferation‚ differentiation commitment‚ and some end-cell functional activation.
Endogenous G-CSF is a lineage-specific colony-stimulating factor that is produced by monocytes‚ fibroblasts, and endothelial cells. G-CSF regulates the production of neutrophils within the bone marrow and affects neutrophil progenitor proliferation‚ differentiation, and selected end-cell functions (including enhanced phagocytic ability‚ priming of the cellular metabolism associated with respiratory burst‚ antibody-dependent killing, and the increased expression of some cell surface antigens). G-CSF is not species-specific and has been shown to have minimal direct in vivo or in vitro effects on the production or activity of hematopoietic cell types other than the neutrophil lineage.
12.2 Pharmacodynamics
In phase 1 studies involving 96 patients with various non-myeloid malignancies‚ filgrastim administration resulted in a dose-dependent increase in circulating neutrophil counts over the dose range of 1 to 70 mcg/kg/day. This increase in neutrophil counts was observed whether filgrastim was administered intravenous (1 to 70 mcg/kg twice daily)‚ subcutaneous (1 to 3 mcg/kg once daily)‚ or by continuous subcutaneous infusion (3 to 11 mcg/kg/day). With discontinuation of filgrastim therapy‚ neutrophil counts returned to baseline in most cases within 4 days. Isolated neutrophils displayed normal phagocytic (measured by zymosan-stimulated chemoluminescence) and chemotactic (measured by migration under agarose using N-formyl-methionyl-leucyl-phenylalanine [fMLP] as the chemotaxin) activity in vitro.
The absolute monocyte count was reported to increase in a dose-dependent manner in most patients receiving filgrastim; however‚ the percentage of monocytes in the differential count remained within the normal range. Absolute counts of both eosinophils and basophils did not change and were within the normal range following administration of filgrastim. Increases in lymphocyte counts following filgrastim administration have been reported in some normal subjects and patients with cancer.
White blood cell (WBC) differentials obtained during clinical trials have demonstrated a shift towards earlier granulocyte progenitor cells (left shift)‚ including the appearance of promyelocytes and myeloblasts‚ usually during neutrophil recovery following the chemotherapy-induced nadir. In addition‚ Dohle bodies‚ increased granulocyte granulation‚ and hypersegmented neutrophils have been observed. Such changes were transient and were not associated with clinical sequelae, nor were they necessarily associated with infection.
12.3 Pharmacokinetics
Filgrastim products exhibit nonlinear pharmacokinetics. Clearance is dependent on filgrastim product concentration and neutrophil count: G-CSF receptor-mediated clearance is saturated by high concentration of filgrastim products and is diminished by neutropenia. In addition, filgrastim products are cleared by the kidney.
Subcutaneous administration of 3.45 mcg/kg and 11.5 mcg/kg of filgrastim resulted in maximum serum concentrations of 4 and 49 ng/mL‚ respectively‚ within 2 to 8 hours. After intravenous administration, the volume of distribution averaged 150 mL/kg and the elimination half-life was approximately 3.5 hours in both normal subjects and cancer subjects. Clearance rates of filgrastim were approximately 0.5 to 0.7 mL/minute/kg. Single parenteral doses or daily intravenous doses‚ over a 14-day period‚ resulted in comparable half-lives. The half-lives were similar for intravenous administration (231 minutes‚ following doses of 34.5 mcg/kg) and for subcutaneous administration (210 minutes‚ following filgrastim dosages of 3.45 mcg/kg). Continuous 24-hour intravenous infusions of 20 mcg/kg over an 11 to 20-day period produced steady-state serum concentrations of filgrastim with no evidence of drug accumulation over the time period investigated. The absolute bioavailability of filgrastim after subcutaneous administration is 60% to 70%.
Specific Populations
Pediatric Patients
The pharmacokinetics of filgrastim in pediatric patients after chemotherapy are similar to those in adult patients receiving the same weight-normalized doses, suggesting no age-related differences in the pharmacokinetics of filgrastim products [see Use in Specific Populations (8.4)].
Renal Impairment
In a study with healthy volunteers, subjects with moderate renal impairment, and subjects with end-stage renal disease (n = 4 per group), higher serum concentrations were observed in subjects with end-stage renal disease. However, dose adjustment in patients with renal impairment is not necessary.
Hepatic Impairment
Pharmacokinetics and pharmacodynamics of filgrastim are similar between subjects with hepatic impairment and healthy subjects (n = 12/group). The study included 10 subjects with mild hepatic impairment (Child-Pugh Class A) and 2 subjects with moderate hepatic impairment (Child-Pugh Class B). Therefore, NIVESTYM dose adjustment for patients with hepatic impairment is not necessary.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of filgrastim or of other filgrastim products.
While available data suggest that a small proportion of patients developed binding antibodies to filgrastim products, the nature and specificity of these antibodies has not been adequately studied. In clinical studies using filgrastim, the incidence of antibodies binding to filgrastim was 3% (11/333). In these 11 patients, no evidence of a neutralizing response was observed using a cell-based bioassay. Because of the low occurrence of anti-drug antibodies, the effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of filgrastim products is unknown.
Cytopenias resulting from an antibody response to exogenous growth factors have been reported on rare occasions in patients treated with other recombinant growth factors.
Nonclinical Toxicology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of filgrastim products has not been studied. Filgrastim failed to induce bacterial gene mutations in either the presence or absence of a drug metabolizing enzyme system. Filgrastim had no observed effect on the fertility of male or female rats at doses up to 500 mcg/kg.
13.2 Animal Toxicology and Pharmacology
Filgrastim was administered to monkeys‚ dogs‚ hamsters‚ rats‚ and mice as part of a nonclinical toxicology program, which included studies up to 1 year duration. In the repeated-dose studies‚ changes observed were attributable to the expected pharmacological actions of filgrastim (i.e.‚ dose-dependent increases in white blood cell counts‚ increased circulating segmented neutrophils‚ and increased myeloid: erythroid ratio in bone marrow). Histopathologic examination of the liver and spleen revealed evidence of ongoing extramedullary granulopoiesis, and dose-related increases in spleen weight were seen in all species. These changes all reversed after discontinuation of treatment.
Clinical Studies
14 CLINICAL STUDIES
14.1 Patients with Cancer Receiving Myelosuppressive Chemotherapy
The safety and efficacy of filgrastim to decrease the incidence of infection‚ as manifested by febrile neutropenia‚ in patients with nonmyeloid malignancies receiving myelosuppressive anti-cancer drugs were established in a randomized‚ double-blind‚ placebo-controlled trial conducted in patients with small cell lung cancer (Study 1).
In Study 1, patients received up to 6 cycles of intravenous chemotherapy including intravenous cyclophosphamide and doxorubicin on day 1; and etoposide on days 1, 2, and 3 of 21 day cycles. Patients were randomized to receive filgrastim (n = 99) at a dose of 230 mcg/m2 (4 to 8 mcg/kg/day) or placebo (n = 111). Study drug was administered subcutaneously daily beginning on day 4, for a maximum of 14 days. A total of 210 patients were evaluable for efficacy and 207 were evaluable for safety. The demographic and disease characteristics were balanced between arms with a median age of 62 (range 31 to 80) years; 64% males; 89% Caucasian; 72% extensive disease and 28% limited disease.
The main efficacy endpoint was the incidence of febrile neutropenia. Febrile neutropenia was defined as an ANC < 1,000/mm3 and temperature > 38.2°C. Treatment with filgrastim resulted in a clinically and statistically significant reduction in the incidence of infection‚ as manifested by febrile neutropenia, 40% for filgrastim-treated patients and 76% for placebo-treated patients (p < 0.001). There were also statistically significant reductions in the incidence and overall duration of infection manifested by febrile neutropenia; the incidence, severity and duration of severe neutropenia (ANC < 500/mm3); the incidence and overall duration of hospital admissions; and the number of reported days of antibiotic use.
14.2 Patients with Acute Myeloid Leukemia Receiving Induction or Consolidation Chemotherapy
The safety and efficacy of filgrastim to reduce the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy treatment of patients with acute myeloid leukemia (AML) was established in a randomized, double-blind‚ placebo-controlled‚ multi-center trial in patients with newly diagnosed, de novo AML (Study 4).
In Study 4 the initial induction therapy consisted of intravenous daunorubicin days 1, 2, and 3; cytosine arabinoside days 1 to 7; and etoposide days 1 to 5. Patients were randomized to receive subcutaneous filgrastim (n = 259) at a dose of 5 mcg/kg/day or placebo (n = 262) from 24 hours after the last dose of chemotherapy until neutrophil recovery (ANC ≥ 1,000/mm3 for 3 consecutive days or ≥ 10,000/mm3 for 1 day) or for a maximum of 35 days. The demographic and disease characteristics were balanced between arms with a median age of 54 (range 16 to 89) years; 54% males; initial white blood cell count (65% < 25,000/mm3 and 27% > 100,000/mm3); 29% unfavorable cytogenetics.
The main efficacy endpoint was median duration of severe neutropenia defined as neutrophil count < 500/mm3. Treatment with filgrastim resulted in a clinically and statistically significant reduction in median number of days of severe neutropenia, filgrastim-treated patients 14 days, placebo-treated patients 19 days (p = 0.0001: difference of 5 days (95% CI: -6.0, -4.0)).
There was a reduction in the median duration of intravenous antibiotic use, filgrastim-treated patients: 15 days versus placebo-treated patients: 18.5 days; a reduction in the median duration of hospitalization, filgrastim-treated patients: 20 days versus placebo-treated patients: 25 days.
There were no statistically significant differences between the filgrastim and the placebo groups in complete remission rate (69% - filgrastim, 68% - placebo), median time to progression of all randomized patients (165 days - filgrastim, 186 days - placebo), or median overall survival (380 days - filgrastim, 425 days - placebo).
14.3 Patients with Cancer Undergoing Bone Marrow Transplantation
The safety and efficacy of filgrastim to reduce the duration of neutropenia in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by autologous bone marrow transplantation was evaluated in 2 randomized controlled trials of patients with lymphoma (Study 6 and Study 9). The safety and efficacy of filgrastim to reduce the duration of neutropenia in patients undergoing myeloablative chemotherapy followed by allogeneic bone marrow transplantation was evaluated in a randomized placebo-controlled trial (Study 10).
In Study 6 patients with Hodgkin's disease received a preparative regimen of intravenous cyclophosphamide, etoposide, and BCNU ("CVP"), and patients with non-Hodgkin's lymphoma received intravenous BCNU, etoposide, cytosine arabinoside and melphalan ("BEAM"). There were 54 patients randomized 1:1:1 to control, filgrastim 10 mcg/kg/day, and filgrastim 30 mcg/kg/day as a 24-hour continuous infusion starting 24 hours after bone marrow infusion for a maximum of 28 days. The median age was 33 (range 17 to 57) years; 56% males; 69% Hodgkin's disease and 31% non-Hodgkin's lymphoma.
The main efficacy endpoint was duration of severe neutropenia ANC < 500/mm3. A statistically significant reduction in the median number of days of severe neutropenia (ANC < 500/mm3) occurred in the filgrastim-treated groups versus the control group (23 days in the control group‚ 11 days in the 10 mcg/kg/day group, and 14 days in the 30 mcg/kg/day group [11 days in the combined treatment groups‚ p = 0.004]).
In Study 9, patients with Hodgkin's disease and non-Hodgkin's lymphoma received a preparative regimen of intravenous cyclophosphamide, etoposide, and BCNU ("CVP"). There were 43 evaluable patients randomized to continuous subcutaneous infusion filgrastim 10 mcg/kg/day (n = 19), filgrastim 30 mcg/kg/day (n = 10) and no treatment (n = 14) starting the day after marrow infusion for a maximum of 28 days. The median age was 33 (range 17 to 56) years; 67% males; 28% Hodgkin's disease and 72% non-Hodgkin's lymphoma.
The main efficacy endpoint was duration of severe neutropenia. There was statistically significant reduction in the median number of days of severe neutropenia (ANC < 500/mm3) in the filgrastim-treated groups versus the control group (21.5 days in the control group versus 10 days in the filgrastim-treated groups, p < 0.001). The number of days of febrile neutropenia was also reduced significantly in this study (13.5 days in the control group versus 5 days in the filgrastim-treated groups‚ p < 0.0001).
In Study 10, 70 patients scheduled to undergo bone marrow transplantation for multiple underlying conditions using multiple preparative regimens were randomized to receive filgrastim 300 mcg/m2/day (n = 33) or placebo (n = 37) days 5 through 28 after marrow infusion. The median age was 18 (range 1 to 45) years, 56% males. The underlying disease was: 67% hematologic malignancy, 24% aplastic anemia, 9% other. A statistically significant reduction in the median number of days of severe neutropenia occurred in the treated group versus the control group (19 days in the control group and 15 days in the treatment group‚ p < 0.001) and time to recovery of ANC to ≥ 500/mm3 (21 days in the control group and 16 days in the treatment group‚ p < 0.001).
14.4 Patients Undergoing Autologous Peripheral Blood Progenitor Cell Collection and Therapy
The safety and efficacy of filgrastim to mobilize autologous peripheral blood progenitor cells for collection by leukapheresis was supported by the experience in uncontrolled trials, and a randomized trial comparing hematopoietic stem cell rescue using filgrastim mobilized autologous peripheral blood progenitor cells to autologous bone marrow (Study 11). Patients in all these trials underwent a similar mobilization/collection regimen: filgrastim was administered for 6 to 7 days‚ in most cases the apheresis procedure occurred on days 5‚ 6, and 7. The dose of filgrastim ranged between 10 to 24 mcg/kg/day and was administered subcutaneously by injection or continuous intravenous infusion.
Engraftment was evaluated in 64 patients who underwent transplantation using filgrastim mobilized autologous hematopoietic progenitor cells in uncontrolled trials. Two of the 64 patients (3%) did not achieve the criteria for engraftment as defined by a platelet count ≥ 20‚000/mm3 by day 28. In clinical trials of filgrastim for the mobilization of hematopoietic progenitor cells‚ filgrastim was administered to patients at doses between 5 to 24 mcg/kg/day after reinfusion of the collected cells until a sustainable ANC (≥ 500/mm3) was reached. The rate of engraftment of these cells in the absence of filgrastim post transplantation has not been studied.
Study 11 was a randomized, unblinded study of patients with Hodgkin's disease or non-Hodgkin's lymphoma undergoing myeloablative chemotherapy‚ 27 patients received filgrastim-mobilized autologous hematopoietic progenitor cells and 31 patients received autologous bone marrow. The preparative regimen was intravenous BCNU, etoposide, cytosine arabinoside and melphalan ("BEAM"). Patients received daily filgrastim 24 hours after stem cell infusion at a dose of 5 mcg/kg/day. The median age was 33 (range 1 to 59) years; 64% males; 57% Hodgkin's disease and 43% non-Hodgkin's lymphoma. The main efficacy endpoint was number of days of platelet transfusions. Patients randomized to filgrastim-mobilized autologous peripheral blood progenitor cells compared to autologous bone marrow had significantly fewer days of platelet transfusions (median 6 vs 10 days).
14.5 Patients with Severe Chronic Neutropenia
The safety and efficacy of filgrastim to reduce the incidence and duration of sequelae of neutropenia (that is fever‚ infections, oropharyngeal ulcers) in symptomatic adult and pediatric patients with congenital neutropenia‚ cyclic neutropenia‚ or idiopathic neutropenia was established in a randomized controlled trial conducted in patients with severe neutropenia (Study 7).
Patients eligible for Study 7 had a history of severe chronic neutropenia documented with an ANC < 500/mm3 on three occasions during a 6-month period, or in patients with cyclic neutropenia 5 consecutive days of ANC < 500/mm3 per cycle. In addition, patients must have experienced a clinically significant infection during the previous 12 months. Patients were randomized to a 4-month observation period followed by filgrastim treatment or immediate filgrastim treatment. The median age was 12 years (range 7 months to 76 years); 46% males; 34% idiopathic, 17% cyclic and 49% congenital neutropenia.
Filgrastim was administered subcutaneously. The dose of filgrastim was determined by the category of neutropenia. Initial dose of filgrastim:
- •
- Idiopathic neutropenia: 3.6 mcg/kg/day
- •
- Cyclic neutropenia: 6 mcg/kg/day
- •
- Congenital neutropenia: 6 mcg/kg/day divided 2 times per day
The dose was increased incrementally to 12 mcg/kg/day divided 2 times per day if there was no response.
The main efficacy endpoint was response to filgrastim treatment. ANC response from baseline (< 500/mm3) was defined as follows:
- •
- Complete response: median ANC > 1,500/mm3
- •
- Partial response: median ANC ≥ 500/mm3 and ≤ 1,500/mm3 with a minimum increase of 100%
- •
- No response: median ANC < 500/mm3
There were 112 of 123 patients who demonstrated a complete or partial response to filgrastim treatment.
Additional efficacy endpoints included a comparison between patients randomized to 4 months of observation and patients receiving filgrastim of the following parameters:
- •
- incidence of infection
- •
- incidence of fever
- •
- duration of fever
- •
- incidence, duration, and severity of oropharyngeal ulcers
- •
- number of days of antibiotic use
The incidence for each of these 5 clinical parameters was lower in the filgrastim arm compared to the control arm for cohorts in each of the 3 major diagnostic categories. An analysis of variance showed no significant interaction between treatment and diagnosis‚ suggesting that efficacy did not differ substantially in the different diseases. Although filgrastim substantially reduced neutropenia in all patient groups‚ in patients with cyclic neutropenia‚ cycling persisted but the period of neutropenia was shortened to 1 day.
How Supplied/Storage and Handling
16 HOW SUPPLIED/STORAGE AND HANDLING
NIVESTYM injection is a clear, colorless, preservative-free solution supplied as:
Vials
Single-dose vials containing 300 mcg/mL of filgrastim-aafi solution. Dispensing packs of 10 vials (NDC 0069-0293-10).
Single-dose vials containing 480 mcg/1.6 mL (300 mcg/mL) of filgrastim-aafi solution. Dispensing packs of 10 vials (NDC 0069-0294-10).
Prefilled Syringes
Single-dose prefilled syringe with BD UltraSafe Plus™ Passive Needle Guard, containing 300 mcg/0.5 mL of filgrastim-aafi solution.
- •
- Pack of 1 prefilled syringe (NDC 0069-0291-01).
- •
- Pack of 10 prefilled syringes (NDC 0069-0291-10).
Single-dose, prefilled syringe with BD UltraSafe Plus™ Passive Needle Guard, containing 480 mcg/0.8 mL of filgrastim-aafi solution.
- •
- Pack of 1 prefilled syringe (NDC 0069-0292-01).
- •
- Pack of 10 prefilled syringes (NDC 0069-0292-10).
The NIVESTYM syringe plunger stopper and needle cover are not made with natural rubber latex [see Dosage and Administration (2.5)].
Storage
Store NIVESTYM in the refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not leave NIVESTYM in direct sunlight. Avoid freezing; if frozen, thaw in the refrigerator before administration. Discard NIVESTYM if frozen more than once. Avoid shaking. Transport via a pneumatic tube has not been studied.
Medication Guide
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Review the steps for direct patient administration with patients and caregivers. Training by the healthcare provider should aim to ensure that patients and caregivers can successfully perform all of the steps in the Instructions for Use of NIVESTYM vial and prefilled syringe, including showing the patient or caregiver how to measure the required dose, particularly if a patient is on a dose other than the entire prefilled syringe. If a patient or caregiver is not able to demonstrate that they can measure the dose and administer the product successfully, you should consider whether the patient is an appropriate candidate for self-administration of NIVESTYM or whether the patient would benefit from a different NIVESTYM presentation. Advise patients of the following risks and potential risks with NIVESTYM:
- •
- Rupture or enlargement of the spleen may occur. Symptoms include left upper quadrant abdominal pain or left shoulder pain. Advise patients to report pain in these areas to their physician immediately [see Warnings and Precautions (5.1)].
- •
- Dyspnea, with or without fever, progressing to Acute Respiratory Distress Syndrome, may occur.
Advise patients to report dyspnea to their physician immediately [see Warnings and Precautions (5.2)]. - •
- Serious allergic reactions may occur, which may be signaled by rash‚ facial edema‚ wheezing‚ dyspnea‚ hypotension‚ or tachycardia. Advise patients to seek immediate medical attention if signs or symptoms of hypersensitivity reaction occur [see Warnings and Precautions (5.3)].
- •
- In patients with sickle cell disease, sickle cell crisis and death have occurred. Discuss potential risks and benefits for patients with sickle cell disease prior to the administration of human granulocyte colony-stimulating factors [see Warnings and Precautions (5.4)].
- •
- Glomerulonephritis may occur. Symptoms include swelling of the face or ankles, dark colored urine or blood in the urine, or a decrease in urine production. Advise patients to report signs or symptoms of glomerulonephritis to their physician immediately [see Warnings and Precautions (5.5)].
- •
- There may be an increased risk of Myelodysplastic Syndrome and/or Acute Myeloid Leukemia in patients with congenital neutropenia who receive filgrastim products and in patients with breast and lung cancer who receive filgrastim products in conjunction with chemotherapy and/or radiation therapy. Symptoms of MDS and AML may include tiredness, fever, and easy bruising or bleeding. Advise patients to report to their physician signs and symptoms of MDS/AML [see Warnings and Precautions (5.8)].
- •
- Cutaneous vasculitis may occur, which may be signaled by purpura or erythema. Advise patients to report signs or symptoms of vasculitis to their physician immediately [see Warnings and Precautions (5.11)].
- •
- Aortitis may occur. Symptoms may include fever, abdominal pain, malaise, back pain, and increased inflammatory markers. Advise patients to report signs and symptoms of aortitis to their physician immediately [see Warnings and Precautions (5.15)].
Instruct patients who self-administer NIVESTYM using the prefilled syringe or single-dose vial of the:
- •
- Importance of following the applicable Instructions for Use.
- •
- Dangers of reusing needles, syringes, or unused portions of single-dose vials.
- •
- Importance of following local requirements for proper disposal of used syringes, needles, and unused vials.
- •
- Importance of informing the healthcare provider if difficulty occurs when measuring or administering partial contents of the NIVESTYM prefilled syringe. If difficulty occurs, use of the NIVESTYM vial may be considered.
- •
- Difference in product concentration of the NIVESTYM prefilled syringe in comparison to the NIVESTYM vial. When switching patients from the NIVESTYM prefilled syringe to the NIVESTYM vial, or vice versa, ensure that patients understand the correct volume to be administered since the concentration of NIVESTYM differs between the prefilled syringe and the vial.
Patient Information | |
What is NIVESTYM? NIVESTYM is a man-made form of granulocyte colony-stimulating factor (G-CSF). G-CSF is a substance produced by the body. It stimulates the growth of neutrophils, a type of white blood cell important in the body's fight against infection. | |
Do not take NIVESTYM if you have had a serious allergic reaction to human G-CSFs such as filgrastim products or pegfilgrastim products. | |
Before you take NIVESTYM, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | |
How will I receive NIVESTYM?
| |
What are the possible side effects of NIVESTYM? NIVESTYM may cause serious side effects, including:
The most common side effects experienced in patients receiving NIVESTYM include:
These are not all the possible side effects of NIVESTYM. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
How should I store NIVESTYM?
Keep NIVESTYM out of the reach of children. | |
General information about the safe and effective use of NIVESTYM. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NIVESTYM for a condition for which it was not prescribed. Do not give NIVESTYM to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about NIVESTYM that is written for healthcare professionals. | |
What are the ingredients in NIVESTYM? Active ingredient: (filgrastim-aafi) Inactive ingredients: acetate, polysorbate 80, sodium, sorbitol, and water for Injection | |
Manufactured by Hospira, Inc., a Pfizer Company, Lake Forest, IL 60045 USA Distributed by Pfizer Labs, division of Pfizer Inc., New York, NY 10001 USA LAB-0935-5.0 |  |
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: March 2023
Instructions For Use
Instructions for Use
NIVESTYM (Neye-ves-tim)
(filgrastim-aafi)
injection
Single-Dose Prefilled Syringe
Important
Read the Patient Information for important information you need to know about NIVESTYM before using this Instructions for Use.
Before you use a NIVESTYM prefilled syringe, read this important information.
Storing your prefilled syringe |
- •
- Store the NIVESTYM prefilled syringe in the refrigerator between 36˚F to 46˚F (2˚C to 8˚C).
- •
- Do not freeze.
- •
- Keep the NIVESTYM prefilled syringe in the original carton to protect from light or physical damage.
- •
- Take the prefilled syringe out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- •
- The NIVESTYM prefilled syringe may be allowed to reach room temperature for up to 24 hours. Throw away (dispose of) any NIVESTYM prefilled syringe that has been left at room temperature for longer than 24 hours.
- •
- After you inject your dose, throw away (dispose of) any unused NIVESTYM left in the prefilled syringe. Do not save unused NIVESTYM in the prefilled syringe for later use.
- •
- Keep NIVESTYM and all medicines out of the reach of children.
Using your prefilled syringe |
- •
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
- •
- You should not inject a dose of NIVESTYM less than 0.3 mL (180 mcg) from a NIVESTYM prefilled syringe. A dose less than 0.3 mL cannot be accurately measured using the NIVESTYM prefilled syringe.
- •
- Make sure the name NIVESTYM appears on the carton and prefilled syringe label.
- •
- Do not use a NIVESTYM prefilled syringe after the expiration date on the label.
- •
- Do not shake the NIVESTYM prefilled syringe.
- •
- The prefilled syringe has a needle guard that needs to be activated to cover the needle after the injection is given. The needle guard will help prevent needle stick injuries to anyone who handles the prefilled syringe.
- •
- Do not remove the needle cover from the prefilled syringe until you are ready to inject.
- •
- Do not use the NIVESTYM prefilled syringe if the needle cover is missing.
- •
- Do not use the prefilled syringe if the carton is open or damaged.
- •
- Do not use a prefilled syringe if it has been dropped on a hard surface. The prefilled syringe may be broken even if you cannot see the break. Use a new prefilled syringe.
Call your healthcare provider if you have any questions.
About the NIVESTYM prefilled syringe | |
NIVESTYM prefilled syringe parts (see Figure A). NIVESTYM 300 mcg/0.5 mL prefilled syringe is shown as an example. | |
Figure A | |
What you need for your injection | |
Included in the carton:
Not included in the carton (see Figure B)
| |
Figure B | |
Preparing the NIVESTYM prefilled syringe | |
Step 1: | Find a clean, well-lit flat work surface. |
Step 2: | Take the carton containing the NIVESTYM prefilled syringe out of the refrigerator and leave it unopened on your work surface for at least 30 minutes so that it reaches room temperature. Put the original carton with any unused prefilled syringes back in the refrigerator.
|
Step 3: | Wash your hands with soap and water. |
Step 4: | Remove the prefilled syringe from the carton by the needle guard only. Do not remove the prefilled syringe by its plunger or needle cover. See Figure C. Check to make sure that the needle guard is covering the barrel of the prefilled syringe. |
Figure C | |
Do not push the needle guard over the needle cover before the injection. This may activate or lock the needle guard. See Figure D that shows a needle guard that has not yet been activated. This is how the prefilled syringe looks before use. | |
Figure D | |
If the needle guard is covering the needle that means it has been activated. See Figure E that shows a needle guard that has been activated. This is how the prefilled syringe looks after use. Do not use the NIVESTYM prefilled syringe. Get another prefilled syringe that has not been activated and is ready to use. | |
Figure E | |
Step 5: | Check the expiration date on the NIVESTYM prefilled syringe. Do not use the NIVESTYM prefilled syringe if the expiration date has passed. |
Step 6: | Inspect the medicine and prefilled syringe. Turn the prefilled syringe so you can see the medicine and markings in the window. Make sure that you look at the medicine only through the viewing window on the prefilled syringe (see Figure F). Do not inspect the medicine through the plastic of the needle guard. Make sure the medicine in the prefilled syringe is clear and colorless. |
Figure F | |
| |
Step 7: | Choose the injection site
|
Figure G | |
| |
Step 8: | Clean your injection site with an alcohol wipe. See Figure H.
|
Figure H | |
Step 9: | Hold the prefilled syringe by the needle guard with the needle cover pointing up. Carefully pull the needle cover straight off and away from your body. Throw away the needle cover. Do not recap the needle. See Figure I. |
Figure I | |
Your healthcare provider has prescribed either a "full" syringe dose or a "partial" syringe dose.
| |
Partial dosing | |
Step 10: | Point the needle up and tap gently until the air rises to the top. See Figure J. |
Figure J | |
Step 11: | Holding the prefilled syringe as shown, slowly push up on the plunger rod to push out the extra air and medicine until the end of the conical base (edge) of the plunger stopper lines up with the syringe marking for your prescribed dose. See Figure K for an example of a dose of 0.3 mL. Your dose may be different than the example shown. |
Figure K | |
Giving the NIVESTYM prefilled syringe injection | |
Step 12: | With one hand, gently pinch a fold of skin at the injection site. Hold the pinch. See Figure L. |
Figure L | |
Step 13: | With your other hand, hold the prefilled syringe like you would hold a pencil. Use a quick "dart-like" motion to insert the needle at a 45 to 90 degree angle into the skin as shown. See Figure M. |
Figure M | |
Step 14: | Using slow and constant pressure, press down on the plunger rod until it reaches the bottom. See Figure N. |
Figure N | |
Step 15: | Keep the plunger rod fully pressed down while you carefully pull the needle straight out from the injection site. See Figure O. |
Figure O | |
Step 16: | As you let go of the plunger rod, the needle guard will automatically slide over the needle until the needle is completely covered and the needle guard locks into place. Do not recap the needle. See Figure P. |
Figure P | |
Step 17: | There may be a small amount of blood at the injection site. You can press a cotton ball or gauze over the injection site and hold it for 10 seconds. Do not rub the injection site. You may cover the injection site with a small adhesive bandage, if needed. See Figure Q. |
Figure Q | |
Step 18: | Throw away (dispose of) the syringe as instructed by your healthcare provider or by following the instructions below. See Figure R. |
Figure R | |
Disposing of (throw away) used NIVESTYM prefilled syringes | |
- •
- Put the used prefilled syringe in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) prefilled syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Hospira, Inc.,
a Pfizer Company
Lake Forest, IL 60045 USA
US License No. 1974
Distributed by Pfizer Labs,
division of Pfizer Inc.,
New York, NY 10001 USA
LAB-0938-6.0
For more information go to www.pfizer.com or call 1-800-438-1985.
Revised: February 2024
Instructions for Use
NIVESTYM (Neye-ves-tim)
(filgrastim-aafi)
injection
Single-Dose Vial
Important
Read the Patient Information for important information you need to know about NIVESTYM before using these Instructions for Use.
Before you use a NIVESTYM vial, read this important information:
Storing your NIVESTYM vial
- •
- Store the vial in the refrigerator between 36˚F to 46˚F (2˚C to 8˚C).
- •
- Do not freeze.
- •
- Keep the vial in the original carton to protect from light or physical damage.
- •
- Take the vial out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- •
- Throw away (dispose of) any vial that has been left at room temperature for longer than 24 hours.
- •
- After you inject your dose, throw away (dispose of) any unused NIVESTYM left in the vial. Do not save unused NIVESTYM in the vial for later use.
Keep NIVESTYM and all medicines out of the reach of children.
Using your vial
- •
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
- •
- Make sure the name NIVESTYM appears on the carton and vial label.
- •
- Only use the vial 1 time. Discard (throw away) the vial with any remaining NIVESTYM liquid.
- •
- Do not use a vial after the expiration date on the label.
- •
- Do not shake the vial.
- •
- Do not use the vial if the medicine is cloudy or discolored or contains flakes or particles.
Call your healthcare provider if you have any questions.
Step 1: Prepare
- A.
- Remove the vial from the refrigerator.
Find a clean, well-lit, flat work surface. Place the vial on your clean work surface for 30 minutes and allow it to reach room temperature before you give an injection.- •
- Do not try to warm the vial by using a heat source such as hot water or microwave.
- •
- Do not leave the vial in direct sunlight.
- •
- Do not shake the vial.
- •
- Use the vial only 1 time.
- B.
- Inspect the vial.
Make sure the medicine in the vial is clear and colorless.- •
- Do not use the vial if:
- o
- The medicine is cloudy or discolored or contains flakes or particles.
- o
- The expiration date printed on the label has passed.
- •
- In all cases, use a new vial and call your healthcare provider.
- C.
- Gather all materials needed for your injection.
Wash your hands thoroughly with soap and water. On your clean, well-lit, flat work surface, place:- •
- 1 Vial
- •
- 1 Disposable syringe and needle
- •
- 2 Alcohol wipes
- •
- 1 Cotton ball or gauze pad
- •
- 1 Adhesive bandage
- •
- Sharps disposal container
- •
- Only use the disposable syringes and needles that your healthcare provider prescribes.
- •
- Only use the syringes and needles 1 time. Throw away (dispose of) any used syringes and needles. See Step 5 Finish, for instructions about how to properly dispose of used syringes and needles.
- •
- You should only use a syringe that is marked in tenths of milliliters (mL).
- •
- Your healthcare provider will show you how to measure the correct dose of NIVESTYM. This dose will be measured in milliliters (mL).
Step 2: Get Ready
- D.
- Take the cap off the vial. Clean the rubber stopper with 1 alcohol wipe.
- E.
- Check the carton containing the needle and syringe. If the carton has been opened or damaged, do not use that needle and syringe. Dispose of (throw away) that needle and syringe in the sharps disposal container.
- F.
- Hold the syringe by the barrel with the needle cap pointing up. Carefully pull the needle cap straight off and away from your body.
Pull back on the plunger and draw air into the syringe that is the same amount (mL) as the dose of NIVESTYM that your healthcare provider prescribed.
Important: Throw away the needle cap into the sharps disposal container. Do not recap the needle.- G.
- Keep the vial on the flat work surface and insert the needle straight down through the rubber stopper. Do not insert the needle through the rubber stopper more than 1 time.
- H.
- Push the plunger down and inject all the air from the syringe into the vial of NIVESTYM.
- I.
- Keep the needle in the vial and turn the vial upside down. Make sure that the NIVESTYM liquid is covering the tip of the needle.
- J.
- Keep the vial upside down and slowly pull back on the plunger to fill the syringe barrel with NIVESTYM to the correct marking amount (mL) of medicine that matches the dose your healthcare provider prescribed.
- K.
- Keep the needle in the vial and check for air bubbles in the syringe. If there are air bubbles, gently tap the syringe barrel with your finger until the air bubbles rise to the top. Slowly push the plunger up to push the air bubbles out of the syringe.
- L.
- Keep the tip of the needle in the liquid and again pull the plunger back to the number on the syringe barrel that matches your dose. Check again for air bubbles. The air in the syringe will not hurt you, but too large an air bubble can reduce your dose of NIVESTYM. If there are still air bubbles, repeat the steps above to remove them.
- M.
- Check again to make sure that you have the correct dose in the syringe. It is important that you use the exact dose prescribed by your healthcare provider. Do not remove the needle from the vial. Lay the vial down on its side with the needle still in the vial.
Step 3: Select and Prepare the Injection Site
- N.
- Prepare and clean your injection site.
You can use:
- •
- Thigh
- •
- Stomach area (abdomen), except for a 2-inch area right around your navel (belly button)
- •
- Upper outer area of your buttocks (only if someone else is giving you the injection)
- •
- Outer area of upper arm (only if someone else is giving you the injection)
Clean your injection site with a clean alcohol wipe.
Let your skin dry. - •
- Do not touch this area again before injecting.
- •
- If you want to use the same injection site, make sure it is not the same spot on the injection site area you used for a previous injection.
- •
- Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks.
Step 4: Subcutaneous (under the skin) injection
- O.
- Remove the prepared syringe and needle from the vial.
- P.
- Pinch your injection site to create a firm surface.
Important: Keep skin pinched while injecting. - Q.
- Hold the pinch. Insert the needle into the skin at a 45 to 90 degree angle.
- R.
- Using slow and constant pressure, push the plunger until it reaches the bottom.
When done gently pull the needle out of the injection site at the same 45 to 90 degree angle used to insert it.
- S.
- Dispose of (throw away) the used needle and syringe.
- •
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles, and syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- T.
- Examine the injection site.
If there is blood, press a cotton ball or gauze pad on your injection site. Do not rub the injection site. Apply an adhesive bandage if needed.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Hospira, Inc.,
a Pfizer Company
Lake Forest, IL 60045 USA
US License No. 1974
Distributed by Pfizer Labs,
division of Pfizer Inc.,
New York, NY 10001 USA
LAB-0937-5.0
For more information go to www.pfizer.com or call 1-800-438-1985.
Revised: August 2023
Other
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
For medical information about NIVESTYM, please visit www.pfizermedinfo.com or call 1-800-438-1985.
Manufactured by:
Hospira, Inc.,
a Pfizer Company
Lake Forest, IL 60045 USA
US License No. 1974
Distributed by Pfizer Labs,
division of Pfizer Inc.,
New York, NY 10001 USA
BD UltraSafe Plus is a trademark of Becton Dickinson and Company.
LAB-0933-9.0
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information. 9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.