INLYTA®
(axitinib)
Find INLYTA® medical information:
Find INLYTA® medical information:
INLYTA® Quick Finder
What is INLYTA?
What is INLYTA?
- in combination with avelumab or pembrolizumab as your first treatment.
- alone when 1 prior drug treatment regimen for your RCC has not worked.
- have high blood pressure
- have thyroid problems
- have liver problems
- have a history of blood clots in your veins or arteries (types of blood vessels), including stroke, heart attack, or change in vision
- have any bleeding problems
- have a history of heart problems, including heart failure
- have an unhealed wound
- plan to have surgery or have had a recent surgery. You should stop taking INLYTA for at least 2 days before planned surgery. See "What are the possible side effects of INLYTA?"
- are pregnant or plan to become pregnant. Taking INLYTA during pregnancy can harm your unborn baby. You should not become pregnant during treatment with INLYTA.
- are able to become pregnant. You should have a pregnancy test before you start treatment with INLYTA. Use effective birth control during treatment and for 1 week after your last dose of INLYTA. Talk to your healthcare provider about birth control methods that you can use to prevent pregnancy during this time.
- are breastfeeding or plan to breastfeed. It is not known if INLYTA passes into your breast milk. Do not breastfeed during treatment and for 2 weeks after your last dose of INLYTA.
- Use effective birth control during treatment and for 1 week after your last dose of INLYTA.
- If your female partner becomes pregnant during your treatment with INLYTA, tell your healthcare provider right away.
Talk with your healthcare provider before you start taking any new medicine. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take INLYTA?
How should I take INLYTA?
- Take INLYTA exactly as prescribed by your healthcare provider.
- Your healthcare provider may change your dose if needed.
- INLYTA can be taken with or without food.
- Take INLYTA 2 times a day about 12 hours apart.
- Swallow INLYTA tablets whole with a glass of water.
- Your healthcare provider should check your blood pressure regularly during treatment with INLYTA.
- If you vomit or miss a dose of INLYTA, take your next dose at your regular time. Do not take two doses at the same time.
- If you take too much INLYTA, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking INLYTA?
What should I avoid while taking INLYTA?
- Do not drink grapefruit juice or eat grapefruit. Grapefruit may increase the amount of INLYTA in your blood.
What are the possible side effects of INLYTA?
What are the possible side effects of INLYTA?
- High blood pressure (hypertension). High blood pressure is common with INLYTA and may sometimes be severe. Your healthcare provider should check your blood pressure regularly during treatment with INLYTA. If you develop blood pressure problems, your healthcare provider may prescribe medicine to treat your high blood pressure, lower your dose, or stop your treatment with INLYTA.
- Blood clots in your veins or arteries. INLYTA can cause blood clots which can be serious, and sometimes lead to death. Get emergency help and call your healthcare provider if you get any of the following symptoms:
- chest pain or pressure
- pain in your arms, back, neck or jaw
- shortness of breath
- numbness or weakness on one side of your body
- trouble talking
- headache
- vision changes
- Bleeding. INLYTA can cause bleeding which can be serious, and sometimes lead to death. Call your healthcare provider right away or get medical help if you develop any of the following signs or symptoms:
- unexpected bleeding or bleeding that lasts a long time, such as:
- unusual bleeding from the gums
- menstrual bleeding or vaginal bleeding that is heavier than normal
- bleeding that is severe or you cannot control
- pink or brown urine
- red or black stools (looks like tar)
- bruises that happen without a known cause or get larger
- cough up blood or blood clots
- vomit blood or your vomit looks like "coffee grounds"
- unexpected pain, swelling, or joint pain
- headaches, feeling dizzy or weak
- Heart failure. Your healthcare provider should check you for signs or symptoms of heart failure regularly during treatment with INLYTA. Heart failure can be serious and can sometimes lead to death. Tell your healthcare provider if you have any of the following symptoms during your treatment with INLYTA:
- tiredness
- swelling of your stomach-area (abdomen), legs or ankles
- shortness of breath
- protruding neck veins
- Tear in your stomach or intestinal wall (perforation). A tear in your stomach or intestinal wall can be serious and can sometimes lead to death. Get medical help right away if you get the following symptoms:
- severe stomach-area (abdominal) pain or stomach-area pain that does not go away
- vomit blood
- red or black stools
- Thyroid gland problems. Your healthcare provider should do blood tests to check your thyroid gland function before and during your treatment with INLYTA. Tell your healthcare provider if you have any of the following symptoms during your treatment with INLYTA:
- tiredness that worsens or that does not go away
- feeling hot or cold
- your voice deepens
- weight gain or weight loss
- hair loss
- muscle cramps and aches
- Risk of wound healing problems. Wounds may not heal properly during INLYTA treatment. Tell your healthcare provider if you plan to have any surgery before starting or during treatment with INLYTA.
- You should stop taking INLYTA at least 2 days before planned surgery.
- Your healthcare provider should tell you when you may start taking INLYTA again after surgery.
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS). A condition called reversible posterior leukoencephalopathy syndrome (RPLS) can happen during treatment with INLYTA. Call your healthcare provider right away if you get:
- headache
- seizures
- weakness
- confusion
- high blood pressure
- blindness or change in vision
- problems thinking
- Protein in your urine. Your healthcare provider should check your urine for protein before and during your treatment with INLYTA. If you develop protein in your urine, your healthcare provider may decrease your dose of INLYTA or stop your treatment.
- Liver problems. Your healthcare provider will do blood tests before and during your treatment with INLYTA. Your healthcare provider may delay or stop your treatment with INLYTA if you develop severe liver problems.
Tell your healthcare provider right way if you have any of the following symptoms:
- yellowing of your skin or the whites of your eyes
- severe nausea or vomiting
- pain on the right side of your stomach area (abdomen)
- dark urine (tea colored)
- bleeding or bruising more easily than normal
- Heart problems. When INLYTA is used with the medicine avelumab, severe heart problems can happen and can lead to death. Your healthcare provider will check you for heart problems during your treatment with INLYTA. Tell your healthcare provider right away or get medical help if you have any of the following symptoms:
- swelling of your stomach-area, legs, hands feet or ankles
- shortness of breath
- nausea or vomiting
- new or worsening chest discomfort, including pain or pressure
- weight gain
- pain or discomfort in your arms, back, neck, or jaw
- breaking out in a cold sweat
- feeling lightheaded or dizzy
- diarrhea
- feeling tired
- high blood pressure
- muscle and bone pain
- nausea
- mouth sores
- rash, redness, itching, or peeling of your skin on your hands and feet
- hoarseness
- decreased appetite
- low levels of thyroid hormone
- rash
- liver problems
- cough
- shortness of breath
- stomach-area (abdomen) pain
- headache
- diarrhea
- feeling tired or weak
- high blood pressure
- liver problems
- low levels of thyroid hormone
- decreased appetite
- rash, redness, itching or peeling of your skin on your hands and feet
- nausea
- mouth sores or swelling of the lining of the mouth, nose, eyes, throat, intestines, or vagina
- hoarseness
- rash
- cough
- constipation
- diarrhea
- high blood pressure
- feeling tired or weak
- decreased appetite
- nausea
- hoarseness
- rash, redness, itching or peeling of your skin on your hands and feet
- decreased weight
- vomiting
- constipation
These are not all of the possible side effects of INLYTA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store INLYTA?
How should I store INLYTA?
Store INLYTA at room temperature between 68°F to 77°F (20°C to 25°C).
Keep INLYTA and all medicines out of the reach of children.
General information about the safe and effective use of INLYTA.
General information about the safe and effective use of INLYTA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use INLYTA for a condition for which it was not prescribed. Do not give INLYTA to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about INLYTA that is written for health professionals.
What are the ingredients in INLYTA?
What are the ingredients in INLYTA?
Active ingredientInactive ingredients
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypertension
Advise patients that hypertension may develop during INLYTA treatment and that blood pressure should be monitored regularly during treatment [see Warnings and Precautions (5.1)].
Arterial/Venous Thromboembolic Events
Advise patients that arterial and venous thromboembolic events have been observed during INLYTA treatment and to inform their doctor if they experience symptoms suggestive of thromboembolic events [see Warnings and Precautions (5.2, 5.3)].
Hemorrhage
Advise patients that INLYTA may increase the risk of bleeding and to promptly inform their doctor of any bleeding episodes [see Warnings and Precautions (5.4)].
Cardiac Failure
Advise patients that cardiac failure may develop during INLYTA treatment and that signs or symptoms of cardiac failure should be regularly monitored for during treatment [see Warnings and Precautions (5.5)].
Gastrointestinal Disorders
Advise patients that gastrointestinal disorders such as diarrhea, nausea, vomiting, and constipation may develop during INLYTA treatment and to seek immediate medical attention if they experience persistent or severe abdominal pain because cases of gastrointestinal perforation and fistula have been reported in patients taking INLYTA [see Warnings and Precautions (5.6) and Adverse Reactions (6.1)].
Abnormal Thyroid Function
Advise patients that abnormal thyroid function may develop during INLYTA treatment and to inform their doctor if symptoms of abnormal thyroid function occur [see Warnings and Precautions (5.7)].
Impaired Wound Healing
Advise patients that INLYTA may impair wound healing. Advise patients to inform their healthcare provider of any planned surgical procedure [see Warnings and Precautions (5.8)].
Reversible Posterior Leukoencephalopathy Syndrome
Advise patients to inform their doctor if they have worsening of neurological function consistent with RPLS (headache, seizure, lethargy, confusion, blindness and other visual and neurologic disturbances) [see Warnings and Precautions (5.9)].
Hepatotoxicity
Inform patients of the signs and symptoms of hepatotoxicity. Advise patients to contact their healthcare provider immediately for signs or symptoms of hepatotoxicity [see Warnings and Precautions (5.11)].
Major Adverse Cardiovascular Events
Advise patients receiving INLYTA in combination with avelumab to contact their healthcare provider immediately for signs or symptoms of cardiovascular events including but not limited to new or worsening chest discomfort, dyspnea, or peripheral edema [see Warnings and Precautions (5.13)].
Embryo-Fetal Toxicity
Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy [see Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with INLYTA and for 1 week after the last dose.
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 1 week following the last dose [see Warnings and Precautions (5.14) and Use in Specific Populations (8.3)].
When INLYTA is used in combination with avelumab or pembrolizumab, refer to the full prescribing information of avelumab or pembrolizumab for pregnancy and contraception information.
Lactation
Advise patients not to breastfeed while taking INLYTA and for 2 weeks after receiving the last dose [see Use in Specific Populations (8.2)].
When INLYTA is used in combination with avelumab or pembrolizumab, refer to the full prescribing information of avelumab or pembrolizumab for lactation information.
Infertility
Advise males and females of reproductive potential that INLYTA may impair fertility [see Use in Specific Populations (8.3)].
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: September 2022 | ||
| PATIENT INFORMATION INLYTA (in-ly-ta) (axitinib) tablets | |||
| Important information: If your healthcare provider prescribes INLYTA for you to be taken with avelumab or pembrolizumab, also read the Medication Guide for avelumab or pembrolizumab. | |||
| What is INLYTA? INLYTA is a prescription medicine used to treat kidney cancer that has spread or cannot be removed by surgery (advanced renal cell carcinoma or RCC):
| |||
Before taking INLYTA, tell your healthcare provider about all of your medical conditions, including if you:
Talk with your healthcare provider before you start taking any new medicine. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. | |||
How should I take INLYTA?
| |||
What should I avoid while taking INLYTA?
| |||
| What are the possible side effects of INLYTA? INLYTA may cause serious side effects, including:
| |||
|
| ||
| |||
|
| ||
| |||
| |||
|
| ||
| |||
| |||
|
| ||
| |||
| |||
|
| ||
| |||
|
| ||
| |||
|
| ||
| The most common side effects of INLYTA with avelumab include: | |||
|
| ||
| The most common side effects of INLYTA with pembrolizumab include: | |||
|
| ||
| The most common side effects of INLYTA when used alone include: | |||
|
| ||
| INLYTA may cause fertility problems in males and females, which may affect your ability to have a child. Talk to your healthcare provider if this is a concern for you. These are not all of the possible side effects of INLYTA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
| How should I store INLYTA? Store INLYTA at room temperature between 68°F to 77°F (20°C to 25°C). Keep INLYTA and all medicines out of the reach of children. | |||
| General information about the safe and effective use of INLYTA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use INLYTA for a condition for which it was not prescribed. Do not give INLYTA to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about INLYTA that is written for health professionals. | |||
| What are the ingredients in INLYTA? Active ingredient: axitinib Inactive ingredients: microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, magnesium stearate, and Opadry II red 32K15441. The Opadry II red 32K15441 film coating contains: lactose monohydrate, HPMC 2910/Hypromellose 15cP, titanium dioxide, triacetin (glycerol triacetate), and red iron oxide. | |||
| LAB-0439-7.0 | ||
| For more information, go to www.inlyta.com or call 8770744-5675 This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com. | |||
Highlights
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use INLYTA safely and effectively. See full prescribing information for INLYTA. INLYTA (axitinib) tablets, for oral administration Initial U.S. Approval: 2012 RECENT MAJOR CHANGES
INDICATIONS AND USAGEINLYTA is a kinase inhibitor indicated:
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS1 mg and 5 mg tablets (3) CONTRAINDICATIONSNone. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (≥20%) are: INLYTA in combination with avelumab: diarrhea, fatigue, hypertension, musculoskeletal pain, nausea, mucositis, palmar-plantar erythrodysesthesia, dysphonia, decreased appetite, hypothyroidism, rash, hepatotoxicity, cough, dyspnea, abdominal pain, and headache. (6.1) INLYTA in combination with pembrolizumab: diarrhea, fatigue/asthenia, hypertension, hepatotoxicity, hypothyroidism, decreased appetite, palmar-plantar erythrodysesthesia, nausea, stomatitis/mucosal inflammation, dysphonia, rash, cough, and constipation. (6.1) INLYTA as a single agent: diarrhea, hypertension, fatigue, decreased appetite, nausea, dysphonia, palmar-plantar erythrodysesthesia (hand-foot) syndrome, weight decreased, vomiting, asthenia, and constipation. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 9/2022 |
Indications and Usage
1 INDICATIONS AND USAGE
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
First-Line Advanced RCC
INLYTA in Combination with Avelumab
The recommended starting dosage of INLYTA is 5 mg orally taken twice daily (12 hours apart) with or without food in combination with avelumab 800 mg administered as an intravenous infusion over 60 minutes every 2 weeks until disease progression or unacceptable toxicity. When INLYTA is used in combination with avelumab, dose escalation of INLYTA above the initial 5 mg dose may be considered at intervals of two weeks or longer. Review the Full Prescribing Information for recommended avelumab dosing information.
INLYTA in Combination with Pembrolizumab
The recommended starting dosage of INLYTA is 5 mg orally twice daily (12 hours apart) with or without food in combination with pembrolizumab 200 mg every 3 weeks or 400 mg every 6 weeks administered as an intravenous infusion over 30 minutes until disease progression or unacceptable toxicity. When INLYTA is used in combination with pembrolizumab, dose escalation of INLYTA above the initial 5 mg dose may be considered at intervals of six weeks or longer. Review the Full Prescribing Information for recommended pembrolizumab dosing information.
2.2 Dose Modification Guidelines
Dose increase or reduction is recommended based on individual safety and tolerability.
Recommended INLYTA dosage increases and reductions are provided in Table 1.
Over the course of treatment, patients who tolerate INLYTA for at least two consecutive weeks with no adverse reactions Grade >2 (according to the Common Toxicity Criteria for Adverse Events [CTCAE]), are normotensive, and are not receiving anti-hypertension medication, may have their dose increased.
| Dose Modification | Dose Regimen |
|---|---|
| Recommended starting dosage | 5 mg twice daily |
| Dosage increase | |
| First dose increase | 7 mg twice daily |
| Second dose increase | 10 mg twice daily |
| Dosage reduction* | |
| First dose reduction† | 3 mg twice daily |
| Second dose reduction | 2 mg twice daily |
Recommended dosage modifications for adverse reactions for INLYTA are provided in Table 2.
| Adverse Reaction | Severity | Dosage Modifications for INLYTA |
|---|---|---|
| Hypertension [see Warnings and Precautions (5.1)] | SBP > 150 mmHg or DBP > 100 mmHg despite antihypertensive treatment |
|
| SBP > 160 mmHg or DBP > 105 mmHg |
| |
| Grade 4 or hypertensive crisis |
| |
| Hemorrhage [see Warnings and Precautions (5.4)] | Grade 3 or 4 |
|
| Cardiac failure [see Warnings and Precautions (5.5)] | Asymptomatic cardiomyopathy (left ventricular ejection fraction greater than 20% but less than 50% below baseline or below the lower limit of normal if baseline was not obtained) |
|
| Clinically manifested congestive heart failure |
| |
| Impaired wound healing [see Warnings and Precautions (5.8)] | Any Grade |
|
| Reversible Posterior Leukoencephalopathy Syndrome [see Warnings and Precautions (5.9)] | Any Grade |
|
| Proteinuria [see Warnings and Precautions (5.10)] | 2 or more grams proteinuria per 24 hours |
|
| Other Adverse Reactions | Grade 3 |
|
| Grade 4 |
|
Table 3 represents additional recommended dosage modifications for adverse reactions when INLYTA is administered in combination with avelumab or pembrolizumab.
See the Full Prescribing Information for additional dosage information for avelumab or pembrolizumab including dose modifications for immune-mediated adverse reactions.
| Treatment | Adverse Reaction | Severity* | Dosage Modifications for INLYTA |
|---|---|---|---|
| ALT = alanine aminotransferase, AST = aspartate aminotransferase, ULN = upper limit normal | |||
| INLYTA in combination with avelumab OR pembrolizumab | Liver enzyme elevations† | ALT or AST at least 3 times ULN but less than 10 times ULN without concurrent total bilirubin at least 2 times ULN |
|
| ALT or AST increases to more than 3 times ULN with concurrent total bilirubin at least 2 times ULN or ALT or AST at least 10 times ULN |
| ||
| Diarrhea | Grade 1–2 |
| |
| Grade 3 |
| ||
| Grade 4 |
| ||
| INLYTA in combination with avelumab | Major Adverse Cardiovascular Events (MACE) | Grade 3 or 4 |
|
2.3 Dosage Modification for Drug Interactions
Strong CYP3A4/5 Inhibitors
The concomitant use of strong CYP3A4/5 inhibitors should be avoided (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, and voriconazole). Selection of an alternate concomitant medication with no or minimal CYP3A4/5 inhibition potential is recommended. Although INLYTA dose adjustment has not been studied in patients receiving strong CYP3A4/5 inhibitors, if a strong CYP3A4/5 inhibitor must be co-administered, a dose decrease of INLYTA by approximately half is recommended, as this dose reduction is predicted to adjust the axitinib area under the plasma concentration vs time curve (AUC) to the range observed without inhibitors. The subsequent doses can be increased or decreased based on individual safety and tolerability. If co-administration of the strong inhibitor is discontinued, the INLYTA dose should be returned (after 3 – 5 half-lives of the inhibitor) to that used prior to initiation of the strong CYP3A4/5 inhibitor [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
2.4 Dosage Modification for Hepatic Impairment
No starting dose adjustment is required when administering INLYTA to patients with mild hepatic impairment (Child-Pugh class A). Based on the pharmacokinetic data, the INLYTA starting dose should be reduced by approximately half in patients with baseline moderate hepatic impairment (Child-Pugh class B). The subsequent doses can be increased or decreased based on individual safety and tolerability. INLYTA has not been studied in patients with severe hepatic impairment (Child-Pugh class C) [see Warnings and Precautions (5.12), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Hypertension
In a controlled clinical study with INLYTA for the treatment of patients with RCC, hypertension was reported in 145/359 patients (40%) receiving INLYTA and 103/355 patients (29%) receiving sorafenib. Grade 3/4 hypertension was observed in 56/359 patients (16%) receiving INLYTA and 39/355 patients (11%) receiving sorafenib. Hypertensive crisis was reported in 2/359 patients (<1%) receiving INLYTA and none of the patients receiving sorafenib. The median onset time for hypertension (systolic blood pressure >150 mmHg or diastolic blood pressure >100 mmHg) was within the first month of the start of INLYTA treatment and blood pressure increases have been observed as early as 4 days after starting INLYTA. Hypertension was managed with standard anti-hypertensive therapy. Discontinuation of INLYTA treatment due to hypertension occurred in 1/359 patients (<1%) receiving INLYTA and none of the patients receiving sorafenib [see Adverse Reactions (6.1)].
Ensure that blood pressure is well-controlled prior to initiating INLYTA. Monitor patients for hypertension and treat as needed with standard anti-hypertensive therapy. Withhold and then dose reduce INLYTA or permanently discontinue based on severity of hypertension [see Dosage and Administration (2.2)].
5.2 Arterial Thromboembolic Events
In clinical trials, arterial thromboembolic events have been reported, including deaths. In a controlled clinical study with INLYTA for the treatment of patients with RCC, Grade 3/4 arterial thromboembolic events were reported in 4/359 patients (1%) receiving INLYTA and 4/355 patients (1%) receiving sorafenib. Fatal cerebrovascular accident was reported in 1/359 patients (<1%) receiving INLYTA and none of the patients receiving sorafenib [see Adverse Reactions (6.1)].
INLYTA has not been studied in patients who had an arterial thromboembolic event within the previous 12 months. In clinical trials with INLYTA, arterial thromboembolic events (including transient ischemic attack, cerebrovascular accident, myocardial infarction, and retinal artery occlusion) were reported in 17/715 patients (2%), with two deaths secondary to cerebrovascular accident.
Permanently discontinue INLYTA if an arterial thromboembolic event occurs during treatment.
5.3 Venous Thromboembolic Events
In clinical trials, venous thromboembolic events have been reported, including deaths. In a controlled clinical study with INLYTA for the treatment of patients with RCC, venous thromboembolic events were reported in 11/359 patients (3%) receiving INLYTA and 2/355 patients (1%) receiving sorafenib. Grade 3/4 venous thromboembolic events were reported in 9/359 patients (3%) receiving INLYTA (including pulmonary embolism, deep vein thrombosis, retinal vein occlusion and retinal vein thrombosis) and 2/355 patients (1%) receiving sorafenib. Fatal pulmonary embolism was reported in 1/359 patients (<1%) receiving INLYTA and none of the patients receiving sorafenib.
INLYTA has not been studied in patients who had a venous thromboembolic event within the previous 6 months. In clinical trials with INLYTA, venous thromboembolic events were reported in 22/715 patients (3%), with two deaths secondary to pulmonary embolism.
Monitor for signs and symptoms of VTE and PE. Withhold INLYTA and then resume at same dose or permanently discontinue based on severity of VTE.
5.4 Hemorrhage
In a controlled clinical study with INLYTA for the treatment of patients with RCC, hemorrhagic events were reported in 58/359 patients (16%) receiving INLYTA and 64/355 patients (18%) receiving sorafenib. Grade 3/4 hemorrhagic events were reported in 5/359 (1%) patients receiving INLYTA (including cerebral hemorrhage, hematuria, hemoptysis, lower gastrointestinal hemorrhage, and melena) and 11/355 (3%) patients receiving sorafenib. Fatal hemorrhage was reported in 1/359 patients (<1%) receiving INLYTA (gastric hemorrhage) and 3/355 patients (1%) receiving sorafenib.
INLYTA has not been studied in patients who have evidence of untreated brain metastasis or recent active gastrointestinal bleeding and should not be used in those patients. Withhold and then dose reduce INLYTA or discontinue based on severity and persistence of hemorrhage.
5.5 Cardiac Failure
In a controlled clinical study with INLYTA for the treatment of patients with RCC, cardiac failure was reported in 6/359 patients (2%) receiving INLYTA and 3/355 patients (1%) receiving sorafenib. Grade 3/4 cardiac failure was observed in 2/359 patients (1%) receiving INLYTA and 1/355 patients (<1%) receiving sorafenib. Fatal cardiac failure was reported in 2/359 patients (1%) receiving INLYTA and 1/355 patients (<1%) receiving sorafenib. Monitor for signs or symptoms of cardiac failure throughout treatment with INLYTA. Management of cardiac failure may require dose reduction, dose interruption or permanent discontinuation of INLYTA [see Dosage and Administration (2.2)].
5.6 Gastrointestinal Perforation and Fistula Formation
In a controlled clinical study with INLYTA for the treatment of patients with RCC, gastrointestinal perforation was reported in 1/359 patients (<1%) receiving INLYTA and none of the patients receiving sorafenib. In clinical trials with INLYTA, gastrointestinal perforation was reported in 5/715 patients (1%), including one death. In addition to cases of gastrointestinal perforation, fistulas were reported in 4/715 patients (1%).
Monitor for symptoms of gastrointestinal perforation or fistula periodically throughout treatment with INLYTA.
5.7 Thyroid Dysfunction
In a controlled clinical study with INLYTA for the treatment of patients with RCC, hypothyroidism was reported in 69/359 patients (19%) receiving INLYTA and 29/355 patients (8%) receiving sorafenib. Hyperthyroidism was reported in 4/359 patients (1%) receiving INLYTA and 4/355 patients (1%) receiving sorafenib. In patients who had thyroid stimulating hormone (TSH) <5 μU/mL before treatment, elevations of TSH to ≥10 μU/mL occurred in 79/245 patients (32%) receiving INLYTA and 25/232 patients (11%) receiving sorafenib [see Adverse Reactions (6.1)].
Monitor thyroid function before initiation of, and periodically throughout, treatment with INLYTA. Treat hypothyroidism and hyperthyroidism according to standard medical practice to maintain euthyroid state.
5.8 Impaired Wound Healing
Impaired wound healing can occur in patients who receive drugs that inhibit the vascular endothelial growth factor (VEGF) signaling pathway. Therefore, INLYTA has the potential to adversely affect wound healing.
Withhold INLYTA for at least 2 days prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. Resume INLYTA at a reduced dose or discontinue based on severity and persistence of the impaired wound healing. The safety of resumption of INLYTA after resolution of wound healing complications has not been established [see Dosage and Administration (2.2)].
5.9 Reversible Posterior Leukoencephalopathy Syndrome
In a controlled clinical study with INLYTA for the treatment of patients with RCC, reversible posterior leukoencephalopathy syndrome (RPLS) was reported in 1/359 patients (<1%) receiving INLYTA and none of the patients receiving sorafenib [see Adverse Reactions (6.1)]. There were two additional reports of RPLS in other clinical trials with INLYTA.
RPLS is a neurological disorder which can present with headache, seizure, lethargy, confusion, blindness and other visual and neurologic disturbances. Mild to severe hypertension may be present. Magnetic resonance imaging is necessary to confirm the diagnosis of RPLS. Permanently discontinue INLYTA in patients developing RPLS. The safety of reinitiating INLYTA therapy in patients previously experiencing RPLS is not known [see Dosage and Administration (2.2)].
5.10 Proteinuria
In a controlled clinical study with INLYTA for the treatment of patients with RCC, proteinuria was reported in 39/359 patients (11%) receiving INLYTA and 26/355 patients (7%) receiving sorafenib. Grade 3 proteinuria was reported in 11/359 patients (3%) receiving INLYTA and 6/355 patients (2%) receiving sorafenib [see Adverse Reactions (6.1)].
Monitoring for proteinuria before initiation of, and periodically throughout, treatment with INLYTA is recommended. For patients who develop moderate to severe proteinuria, withhold and then dose reduce INLYTA [see Dosage and Administration (2.2)].
5.11 Hepatotoxicity
INLYTA as a Single Agent
In a controlled clinical study with INLYTA for the treatment of patients with RCC, alanine aminotransferase (ALT) elevations of all grades occurred in 22% of patients on both arms, with Grade 3/4 events in <1% of patients on the INLYTA arm. When used as a single agent, monitor ALT, aspartate aminotransferase (AST) and bilirubin before initiation of and periodically throughout treatment with INLYTA.
INLYTA in Combination with Avelumab or with Pembrolizumab
INLYTA in combination with avelumab or with pembrolizumab can cause hepatotoxicity with higher than expected frequencies of Grade 3 and 4 ALT and AST elevations. Monitor liver enzymes before initiation of and periodically throughout treatment. Consider more frequent monitoring of liver enzymes as compared to when the drugs are administered as single agents. For elevated liver enzymes, interrupt or permanently discontinue INLYTA and avelumab or pembrolizumab, and administer corticosteroids as needed [see Dosage and Administration (2.3)].
With the combination of INLYTA and avelumab, Grades 3 and 4 increased ALT and increased AST were reported in 9% and 7% of patients, respectively. In patients with ALT ≥3 times ULN (Grades 2–4, n=82), ALT resolved to Grades 0–1 in 92%. Among the 73 patients who were rechallenged with either avelumab (n=3) or axitinib (n=25) administered as a single agent or with both (n=45), recurrence of ALT ≥3 times ULN was observed in no patient receiving avelumab, 6 patients receiving INLYTA, and 15 patients receiving both avelumab and INLYTA. Twenty-two (88%) patients with a recurrence of ALT ≥3 ULN subsequently recovered to Grade 0–1 from the event. Immune-mediated hepatitis was reported in 7% of patients including 4.9% with Grade 3 or 4 immune-mediated hepatitis. Hepatotoxicity led to permanent discontinuation in 7% and immune-mediated hepatitis led to permanent discontinuation of either avelumab or INLYTA in 5% of patients. Thirty-four patients were treated with corticosteroids and one patient was treated with a non-steroidal immunosuppressant. Resolution of hepatitis occurred in 31 of the 35 patients at the time of data cut-off.
With the combination of INLYTA and pembrolizumab, Grades 3 and 4 increased ALT (20%) and increased AST (13%) were seen. Fifty-nine percent of the patients with increased ALT received systemic corticosteroids. In patients with ALT ≥3 times ULN (Grades 2–4, n=116), ALT resolved to Grades 0–1 in 94%. Among the 92 patients who were rechallenged with either pembrolizumab (n=3) or INLYTA (n=34) administered as a single agent or with both (n=55), recurrence of ALT ≥3 times ULN was observed in 1 patient receiving pembrolizumab, 16 patients receiving INLYTA, and 24 patients receiving both pembrolizumab and INLYTA. All patients with a recurrence of ALT ≥3 ULN subsequently recovered from the event.
5.12 Use in Patients with Hepatic Impairment
The systemic exposure to axitinib was higher in subjects with moderate hepatic impairment (Child-Pugh class B) compared to subjects with normal hepatic function. A dose decrease is recommended when administering INLYTA to patients with moderate hepatic impairment (Child-Pugh class B). INLYTA has not been studied in patients with severe hepatic impairment (Child-Pugh class C) [see Dosage and Administration (2.2), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
5.13 Major Adverse Cardiovascular Events (MACE)
INLYTA in combination with avelumab can cause severe and fatal cardiovascular events. Consider baseline and periodic evaluations of left ventricular ejection fraction. Monitor for signs and symptoms of cardiovascular events. Optimize management of cardiovascular risk factors, such as hypertension, diabetes, or dyslipidemia. Permanently discontinue INLYTA and avelumab for Grade 3–4 cardiovascular events.
MACE occurred in 7% of patients with advanced RCC treated with INLYTA in combination with avelumab compared to 3.4% treated with sunitinib in a randomized trial, JAVELIN Renal 101. These events included death due to cardiac events (1.4%), Grade 3–4 myocardial infarction (2.8%), and Grade 3–4 congestive heart failure (1.8%). Median time to onset of MACE was 4.2 months (range: 2 days to 24.5 months).
5.14 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies, INLYTA can cause fetal harm when administered to a pregnant woman. There are no available human data to inform the drug-associated risk. In developmental toxicity studies in mice, axitinib was teratogenic, embryotoxic and fetotoxic at maternal exposures that were lower than human exposures at the recommended clinical dose. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception during treatment with INLYTA and for 1 week after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with INLYTA and for 1 week after the last dose [see Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1)].
When INLYTA is used in combination with avelumab or pembrolizumab, refer to the full prescribing information of avelumab or pembrolizumab for pregnancy and contraception information.
Adverse Reactions
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed elsewhere in the labeling [see Warnings and Precautions (5)]:
- Hypertension [see Warnings and Precautions (5.1)]
- Arterial thromboembolic events [see Warnings and Precautions (5.2)]
- Venous thromboembolic events [see Warnings and Precautions (5.3)]
- Hemorrhage [see Warnings and Precautions (5.4)]
- Cardiac failure [see Warnings and Precautions (5.5)]
- Gastrointestinal perforation and fistula formation [see Warnings and Precautions (5.6)]
- Thyroid dysfunction [see Warnings and Precautions (5.7)]
- Reversible posterior leukoencephalopathy syndrome [see Warnings and Precautions (5.9)]
- Proteinuria [see Warnings and Precautions (5.10)]
- Hepatotoxicity [see Warnings and Precautions (5.11)]
- Hepatic impairment [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of INLYTA has been evaluated in combination with avelumab in JAVELIN Renal 101 and pembrolizumab in KEYNOTE-426 for the first-line treatment of patients with advanced RCC [see Clinical Studies (14.1)]. The data described [see Adverse Reactions (6.1)] reflect exposure to INLYTA in combination with avelumab in 434 patients and pembrolizumab in 429 patients [see Clinical Studies (14.1)].
The safety of INLYTA has been evaluated in 715 patients in second-line monotherapy studies, which included 537 patients with advanced RCC. The data described [see Adverse Reactions (6.1)] reflect exposure to INLYTA in 359 patients with advanced RCC who participated in a randomized clinical study versus sorafenib [see Clinical Studies (14.2)].
First-Line Advanced RCC
INLYTA in Combination with Avelumab
The safety of INLYTA in combination with avelumab was evaluated in JAVELIN Renal 101. Patients with autoimmune disease other than type I diabetes mellitus, vitiligo, psoriasis, or thyroid disorders not requiring immunosuppressive treatment were excluded. Patients received INLYTA 5 mg twice daily (N=434) in combination with avelumab 10 mg/kg every 2 weeks administered or sunitinib 50 mg once daily for 4 weeks followed by 2 weeks off (N=439).
In the INLYTA plus avelumab arm, 70% were exposed to avelumab for ≥6 months and 29% were exposed for ≥1 year in JAVELIN Renal 101 [see Clinical Studies (14.1)].
The median age of patients treated with INLYTA in combination with avelumab was 62 years (range: 29 to 83), 38% of patients were 65 years or older, 71% were male, 75% were White, and the Eastern Cooperative Oncology Group (ECOG) performance score was 0 (64%) or 1 (36%).
Fatal adverse reactions occurred in 1.8% of patients receiving INLYTA in combination with avelumab. These included sudden cardiac death (1.2%), stroke (0.2%), myocarditis (0.2%), and necrotizing pancreatitis (0.2%).
Serious adverse reactions occurred in 35% of patients receiving INLYTA in combination with avelumab. Serious adverse reactions in ≥1% of patients included diarrhea (2.5%), dyspnea (1.8%), hepatotoxicity (1.8%), venous thromboembolic disease (1.6%), acute kidney injury (1.4%), and pneumonia (1.2%).
Permanent discontinuation due to an adverse reaction of either INLYTA or avelumab occurred in 22% of patients: 19% avelumab only, 13% INLYTA only, and 8% both drugs. The most common adverse reactions (>1%) resulting in permanent discontinuation of avelumab or the combination were hepatotoxicity (6%) and infusion-related reaction (1.8%).
Dose interruptions or reductions due to an adverse reaction, excluding temporary interruptions of avelumab infusions due to infusion-related reactions, occurred in 76% of patients receiving INLYTA in combination with avelumab. This includes interruption of avelumab in 50% of patients. INLYTA was interrupted in 66% and dose reduced in 19% of patients. The most common adverse reaction (>10%) resulting in interruption of avelumab was diarrhea (10%). The most common adverse reactions resulting in either interruption or dose reduction of INLYTA were diarrhea (19%), hypertension (18%), palmar-plantar erythrodysesthesia (18%), and hepatotoxicity (10%).
The most common adverse reactions (≥20%) in patients receiving INLYTA in combination with avelumab were diarrhea, fatigue, hypertension, musculoskeletal pain, nausea, mucositis, palmar-plantar erythrodysesthesia, dysphonia, decreased appetite, hypothyroidism, rash, hepatotoxicity, cough, dyspnea, abdominal pain, and headache.
Forty-eight (11%) of patients treated with INLYTA in combination with avelumab received an oral prednisone dose equivalent to ≥40 mg daily for an immune-mediated adverse reaction [see Warnings and Precautions (5.12)].
Table 4 summarizes adverse reactions that occurred in ≥20% of INLYTA in combination with avelumab-treated patients.
| Adverse Reactions | INLYTA plus Avelumab (N=434) | Sunitinib (N=439) | ||
|---|---|---|---|---|
| All Grades % | Grade 3–4 % | All Grades % | Grade 3–4 % | |
| Toxicity was graded per National Cancer Institute Common Terminology Criteria for Adverse Events. Version 4.03 (NCI CTCAE v4). | ||||
| ||||
| Gastrointestinal Disorders | ||||
| Diarrhea† | 62 | 8 | 48 | 2.7 |
| Nausea | 34 | 1.4 | 39 | 1.6 |
| Mucositis‡ | 34 | 2.8 | 35 | 2.1 |
| Hepatotoxicity§ | 24 | 9 | 18 | 3.6 |
| Abdominal pain¶ | 22 | 1.4 | 19 | 2.1 |
| General Disorders and Administration Site Conditions | ||||
| Fatigue# | 53 | 6 | 54 | 6 |
| Vascular Disorders | ||||
| HypertensionÞ | 50 | 26 | 36 | 17 |
| Musculoskeletal and Connective Tissue Disorders | ||||
| Musculoskeletal painß | 40 | 3.2 | 33 | 2.7 |
| Skin and Subcutaneous Tissue Disorders | ||||
| Palmar-plantar erythrodysesthesia | 33 | 6 | 34 | 4 |
| Rashà | 25 | 0.9 | 16 | 0.5 |
| Respiratory, Thoracic and Mediastinal Disorders | ||||
| Dysphonia | 31 | 0.5 | 3.2 | 0 |
| Dyspneaè | 23 | 3.0 | 16 | 1.8 |
| Cough | 23 | 0.2 | 19 | 0 |
| Metabolism and Nutrition Disorders | ||||
| Decreased appetite | 26 | 2.1 | 29 | 0.9 |
| Endocrine Disorders | ||||
| Hypothyroidism | 25 | 0.2 | 14 | 0.2 |
| Nervous System Disorders | ||||
| Headache | 21 | 0.2 | 16 | 0.2 |
Other clinically important adverse reactions that occurred in less than 20% of patients in JAVELIN Renal 101 included arthralgia, weight decreased, and chills.
Patients received pre-medication with an anti-histamine and acetaminophen prior to each infusion. Infusion-related reactions occurred in 12% (Grade 3: 1.6%; no Grade 4) of patients treated with INLYTA in combination with avelumab.
Table 5 summarizes selected laboratory abnormalities that occurred in ≥20% of INLYTA in combination with avelumab-treated patients.
| Laboratory Abnormality | INLYTA plus Avelumab | Sunitinib† | ||
|---|---|---|---|---|
| Any Grade % | Grade 3–4 % | Any Grade % | Grade 3–4 % | |
| ||||
| Chemistry | ||||
| Blood triglycerides increased | 71 | 13 | 48 | 5 |
| Blood creatinine increased | 62 | 2.3 | 68 | 1.4 |
| Blood cholesterol increased | 57 | 1.9 | 22 | 0.7 |
| Alanine aminotransferase increased (ALT) | 50 | 9 | 46 | 3.2 |
| Aspartate aminotransferase increased (AST) | 47 | 7 | 57 | 3.2 |
| Blood sodium decreased | 38 | 9 | 37 | 10 |
| Lipase increased | 37 | 14 | 25 | 7 |
| Blood potassium increased | 35 | 3.0 | 28 | 3.9 |
| Blood bilirubin increased | 21 | 1.4 | 23 | 1.4 |
| Hematology | ||||
| Platelet count decreased | 27 | 0.7 | 80 | 1.5 |
| Hemoglobin decreased | 21 | 2.1 | 65 | 8 |
INLYTA in Combination with Pembrolizumab
The safety of INLYTA in combination with pembrolizumab was investigated in KEYNOTE-426 [see Clinical Studies (14.1)]. Patients with medical conditions that required systemic corticosteroids or other immunosuppressive medications or had a history of severe autoimmune disease other than type 1 diabetes, vitiligo, Sjogren's syndrome, and hypothyroidism stable on hormone replacement were ineligible. Patients received INLYTA 5 mg orally twice daily and pembrolizumab 200 mg intravenously every 3 weeks, or sunitinib 50 mg once daily for 4 weeks and then off treatment for 2 weeks. The median duration of exposure to the combination therapy of INLYTA and pembrolizumab was 10.4 months (range: 1 day to 21.2 months).
The study population characteristics were: median age of 62 years (range: 30 to 89), 40% age 65 or older; 71% male; 80% White; and 80% Karnofsky Performance Status (KPS) of 90–100 and 20% KPS of 70–80.
Fatal adverse reactions occurred in 3.3% of patients receiving INLYTA in combination with pembrolizumab. These included 3 cases of cardiac arrest, 2 cases of pulmonary embolism and 1 case each of cardiac failure, death due to unknown cause, myasthenia gravis, myocarditis, Fournier's gangrene, plasma cell myeloma, pleural effusion, pneumonitis, and respiratory failure.
Serious adverse reactions occurred in 40% of patients receiving INLYTA in combination with pembrolizumab. Serious adverse reactions in ≥1% of patients receiving INLYTA in combination with pembrolizumab included hepatotoxicity (7%), diarrhea (4.2%), acute kidney injury (2.3%), dehydration (1%), and pneumonitis (1%).
Permanent discontinuation due to an adverse reaction of either INLYTA or pembrolizumab occurred in 31% of patients; 13% pembrolizumab only, 13% INLYTA only, and 8% both drugs. The most common adverse reaction (>1%) resulting in permanent discontinuation of INLYTA, pembrolizumab, or the combination was hepatotoxicity (13%), diarrhea/colitis (1.9%), acute kidney injury (1.6%), and cerebrovascular accident (1.2%).
Dose interruptions or reductions due to an adverse reaction, excluding temporary interruptions of pembrolizumab infusions due to infusion-related reactions, occurred in 76% of patients receiving pembrolizumab in combination with INLYTA. This includes interruption of pembrolizumab in 50% of patients. INLYTA was interrupted in 64% of patients and dose reduced in 22% of patients. The most common adverse reactions (>10%) resulting in either interruption or reduction of INLYTA were hepatotoxicity (21%), diarrhea (19%), and hypertension (18%) and the most common adverse reactions (>10%) resulting in interruption of pembrolizumab were hepatotoxicity (14%) and diarrhea (11%).
The most common adverse reactions (≥20%) in patients receiving INLYTA and pembrolizumab were diarrhea, fatigue/asthenia, hypertension, hepatotoxicity, hypothyroidism, decreased appetite, palmar-plantar erythrodysesthesia, nausea, stomatitis/mucosal inflammation, dysphonia, rash, cough, and constipation.
Twenty-seven percent (27%) of patients treated with INLYTA in combination with pembrolizumab received an oral prednisone dose equivalent to ≥40 mg daily for an immune-mediated adverse reaction.
Tables 6 and 7 summarize the adverse reactions and laboratory abnormalities, respectively, that occurred in at least 20% of patients treated with INLYTA and pembrolizumab in KEYNOTE-426.
| Adverse Reactions | INLYTA plus Pembrolizumab N=429 | Sunitinib N=425 | ||
|---|---|---|---|---|
| All Grades* % | Grades 3–4 % | All Grades % | Grades 3–4 % | |
| ||||
| Gastrointestinal Disorders | ||||
| Diarrhea† | 56 | 11 | 45 | 5 |
| Nausea | 28 | 0.9 | 32 | 0.9 |
| Constipation | 21 | 0 | 15 | 0.2 |
| General | ||||
| Fatigue/Asthenia | 52 | 5 | 51 | 10 |
| Vascular | ||||
| Hypertension‡ | 48 | 24 | 48 | 20 |
| Hepatobiliary | ||||
| Hepatotoxicity§ | 39 | 20 | 25 | 4.9 |
| Endocrine | ||||
| Hypothyroidism | 35 | 0.2 | 32 | 0.2 |
| Metabolism and Nutrition | ||||
| Decreased appetite | 30 | 2.8 | 29 | 0.7 |
| Skin and Subcutaneous Tissue | ||||
| Palmar-plantar erythrodysesthesia syndrome | 28 | 5 | 40 | 3.8 |
| Stomatitis/Mucosal inflammation | 27 | 1.6 | 41 | 4 |
| Rash¶ | 25 | 1.4 | 21 | 0.7 |
| Respiratory, Thoracic, and Mediastinal | ||||
| Dysphonia | 25 | 0.2 | 3.3 | 0 |
| Cough | 21 | 0.2 | 14 | 0.5 |
| Laboratory Test* | INLYTA plus Pembrolizumab | Sunitinib | ||
|---|---|---|---|---|
| All Grades† % | Grade 3–4 % | All Grades % | Grade 3–4 % | |
| ||||
| Chemistry | ||||
| Hyperglycemia | 62 | 9 | 54 | 3.2 |
| Increased ALT | 60 | 20 | 44 | 5 |
| Increased AST | 57 | 13 | 56 | 5 |
| Increased creatinine | 43 | 4.3 | 40 | 2.4 |
| Hyponatremia | 35 | 8 | 29 | 8 |
| Hyperkalemia | 34 | 6 | 22 | 1.7 |
| Hypoalbuminemia | 32 | 0.5 | 34 | 1.7 |
| Hypercalcemia | 27 | 0.7 | 15 | 1.9 |
| Hypophosphatemia | 26 | 6 | 49 | 17 |
| Increased alkaline phosphatase | 26 | 1.7 | 30 | 2.7 |
| Hypocalcemia‡ | 22 | 0.2 | 29 | 0.7 |
| Blood bilirubin increased | 22 | 2.1 | 21 | 1.9 |
| Activated partial thromboplastin time prolonged§ | 22 | 1.2 | 14 | 0 |
| Hematology | ||||
| Lymphopenia | 33 | 11 | 46 | 8 |
| Anemia | 29 | 2.1 | 65 | 8 |
| Thrombocytopenia | 27 | 1.4 | 78 | 14 |
Second-Line Advanced RCC
The median duration of treatment was 6.4 months (range 0.03 to 22.0) for patients who received INLYTA and 5.0 months (range 0.03 to 20.1) for patients who received sorafenib. Dose modifications or temporary delay of treatment due to an adverse reaction occurred in 199/359 patients (55%) receiving INLYTA and 220/355 patients (62%) receiving sorafenib. Permanent discontinuation due to an adverse reaction occurred in 34/359 patients (9%) receiving INLYTA and 46/355 patients (13%) receiving sorafenib.
The most common (≥20%) adverse reactions observed following treatment with INLYTA were diarrhea, hypertension, fatigue, decreased appetite, nausea, dysphonia, palmar-plantar erythrodysesthesia (hand-foot) syndrome, weight decreased, vomiting, asthenia, and constipation. Table 8 presents adverse reactions reported in ≥10% patients who received INLYTA or sorafenib.
| Adverse Reaction* | INLYTA | Sorafenib | ||
|---|---|---|---|---|
| (N=359) | (N=355) | |||
| All Grades† | Grade 3/4 | All Grades† | Grade 3/4 | |
| % | % | % | % | |
| Diarrhea | 55 | 11 | 53 | 7 |
| Hypertension | 40 | 16 | 29 | 11 |
| Fatigue | 39 | 11 | 32 | 5 |
| Decreased appetite | 34 | 5 | 29 | 4 |
| Nausea | 32 | 3 | 22 | 1 |
| Dysphonia | 31 | 0 | 14 | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 27 | 5 | 51 | 16 |
| Weight decreased | 25 | 2 | 21 | 1 |
| Vomiting | 24 | 3 | 17 | 1 |
| Asthenia | 21 | 5 | 14 | 3 |
| Constipation | 20 | 1 | 20 | 1 |
| Hypothyroidism | 19 | <1 | 8 | 0 |
| Cough | 15 | 1 | 17 | 1 |
| Mucosal inflammation | 15 | 1 | 12 | 1 |
| Arthralgia | 15 | 2 | 11 | 1 |
| Stomatitis | 15 | 1 | 12 | <1 |
| Dyspnea | 15 | 3 | 12 | 3 |
| Abdominal pain | 14 | 2 | 11 | 1 |
| Headache | 14 | 1 | 11 | 0 |
| Pain in extremity | 13 | 1 | 14 | 1 |
| Rash | 13 | <1 | 32 | 4 |
| Proteinuria | 11 | 3 | 7 | 2 |
| Dysgeusia | 11 | 0 | 8 | 0 |
| Dry skin | 10 | 0 | 11 | 0 |
| Dyspepsia | 10 | 0 | 2 | 0 |
| Pruritus | 7 | 0 | 12 | 0 |
| Alopecia | 4 | 0 | 32 | 0 |
| Erythema | 2 | 0 | 10 | <1 |
Selected adverse reactions (all grades) that were reported in <10% of patients treated with INLYTA included dizziness (9%), upper abdominal pain (8%), myalgia (7%), dehydration (6%), epistaxis (6%), anemia (4%), hemorrhoids (4%), hematuria (3%), tinnitus (3%), lipase increased (3%), glossodynia (3%), pulmonary embolism (2%), rectal hemorrhage (2%), hemoptysis (2%), deep vein thrombosis (1%), retinal-vein occlusion/thrombosis (1%), polycythemia (1%), and transient ischemic attack (1%).
Table 9 presents the most common laboratory abnormalities reported in ≥10% patients who received INLYTA or sorafenib.
| Laboratory Abnormality | N | INLYTA | N | Sorafenib | ||
|---|---|---|---|---|---|---|
| All Grades* | Grade 3/4 | All Grades* | Grade 3/4 | |||
| % | % | % | % | |||
| ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase | ||||||
| ||||||
| Hematology | ||||||
| Hemoglobin decreased | 320 | 35 | <1 | 316 | 52 | 4 |
| Lymphocytes (absolute) decreased | 317 | 33 | 3 | 309 | 36 | 4 |
| Platelets decreased | 312 | 15 | <1 | 310 | 14 | 0 |
| White blood cells decreased | 320 | 11 | 0 | 315 | 16 | <1 |
| Chemistry | ||||||
| Creatinine increased | 336 | 55 | 0 | 318 | 41 | <1 |
| Bicarbonate decreased | 314 | 44 | <1 | 291 | 43 | 0 |
| Hypocalcemia | 336 | 39 | 1 | 319 | 59 | 2 |
| ALP increased | 336 | 30 | 1 | 319 | 34 | 1 |
| Hyperglycemia | 336 | 28 | 2 | 319 | 23 | 2 |
| Lipase increased | 338 | 27 | 5 | 319 | 46 | 15 |
| Amylase increased | 338 | 25 | 2 | 319 | 33 | 2 |
| ALT increased | 331 | 22 | <1 | 313 | 22 | 2 |
| AST increased | 331 | 20 | <1 | 311 | 25 | 1 |
| Hypernatremia | 338 | 17 | 1 | 319 | 13 | 1 |
| Hypoalbuminemia | 337 | 15 | <1 | 319 | 18 | 1 |
| Hyperkalemia | 333 | 15 | 3 | 314 | 10 | 3 |
| Hypoglycemia | 336 | 11 | <1 | 319 | 8 | <1 |
| Hyponatremia | 338 | 13 | 4 | 319 | 11 | 2 |
| Hypophosphatemia | 336 | 13 | 2 | 318 | 49 | 16 |
Selected laboratory abnormalities (all grades) that were reported in <10% of patients treated with INLYTA included hemoglobin increased (above the upper limit of normal) (9% for INLYTA versus 1% for sorafenib) and hypercalcemia (6% for INLYTA versus 2% for sorafenib).
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of INLYTA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Vascular disorders: arterial (including aortic), aneurysms, dissections, and rupture.
Drug Interactions
7 DRUG INTERACTIONS
7.1 CYP3A4/5 Inhibitors
Co-administration of ketoconazole, a strong inhibitor of CYP3A4/5, increased the plasma exposure of axitinib in healthy volunteers. Co-administration of INLYTA with strong CYP3A4/5 inhibitors should be avoided. Grapefruit or grapefruit juice may also increase axitinib plasma concentrations and should be avoided. Selection of concomitant medication with no or minimal CYP3A4/5 inhibition potential is recommended. If a strong CYP3A4/5 inhibitor must be co-administered, the INLYTA dose should be reduced [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
7.2 CYP3A4/5 Inducers
Co-administration of rifampin, a strong inducer of CYP3A4/5, reduced the plasma exposure of axitinib in healthy volunteers. Co-administration of INLYTA with strong CYP3A4/5 inducers (e.g., rifampin, dexamethasone, phenytoin, carbamazepine, rifabutin, rifapentine, phenobarbital, and St. John's wort) should be avoided. Selection of concomitant medication with no or minimal CYP3A4/5 induction potential is recommended [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)]. Moderate CYP3A4/5 inducers (e.g., bosentan, efavirenz, etravirine, modafinil, and nafcillin) may also reduce the plasma exposure of axitinib and should be avoided if possible.
Use in Specific Populations
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animal studies and its mechanism of action, INLYTA can cause fetal harm when administered to a pregnant woman. There are no available human data to inform the drug-associated risk. In developmental toxicity studies, axitinib was teratogenic, embryotoxic and fetotoxic in mice at exposures lower than human exposures at the recommended starting dose (see Data). Advise females of reproductive potential of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated populations are unknown. However, the background risk in the United States (U.S.) general population of major birth defects is 2%–4% and of miscarriage is 15%–20% of clinically recognized pregnancies.
When INLYTA is used in combination with avelumab or pembrolizumab, refer to the full prescribing information of avelumab or pembrolizumab for pregnancy information.
Data
Animal Data
Oral axitinib administered twice daily to female mice prior to mating and through the first week of pregnancy caused an increase in post-implantation loss at all doses tested (≥15 mg/kg/dose, approximately 10 times the systemic exposure (AUC) in patients at the recommended starting dose). In an embryo-fetal developmental toxicity study, pregnant mice received oral doses of 0.15, 0.5 and 1.5 mg/kg/dose axitinib twice daily during the period of organogenesis. Embryo-fetal toxicities observed in the absence of maternal toxicity included malformation (cleft palate) at 1.5 mg/kg/dose (approximately 0.5 times the AUC in patients at the recommended starting dose) and variation in skeletal ossification at ≥0.5 mg/kg/dose (approximately 0.15 times the AUC in patients at the recommended starting dose).
8.2 Lactation
Risk Summary
There are no data on the presence of axitinib in human milk, or its effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in a breastfed child from INLYTA, advise lactating women not to breastfeed during treatment and for 2 weeks after the last dose.
When INLYTA is used in combination with avelumab or pembrolizumab, refer to the full prescribing information of avelumab or pembrolizumab for lactation information.
8.3 Females and Males of Reproductive Potential
Based on findings in animal studies, INLYTA can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. When INLYTA is used in combination with avelumab or pembrolizumab, refer to the full prescribing information of avelumab or pembrolizumab for contraception information.
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating treatment with INLYTA.
Contraception
Infertility
Females and Males
Based on findings in animals, INLYTA may impair fertility in females and males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and efficacy of INLYTA in pediatric patients have not been studied.
Juvenile Animal Toxicity Data
Toxicities in bone and teeth were observed in immature mice and dogs administered oral axitinib twice daily for 1 month or longer. Effects in bone consisted of thickened growth plates in mice and dogs at ≥15 mg/kg/dose (approximately 6 and 15 times, respectively, the systemic exposure (AUC) in patients at the recommended starting dose). Abnormalities in growing incisor teeth (including dental caries, malocclusions and broken and/or missing teeth) were observed in mice administered oral axitinib twice daily at ≥5 mg/kg/dose (approximately 1.5 times the AUC in patients at the recommended starting dose). Other toxicities of potential concern to pediatric patients have not been evaluated in juvenile animals.
8.5 Geriatric Use
In a controlled clinical study with INLYTA for the treatment of patients with RCC, 123/359 patients (34%) treated with INLYTA were ≥65 years of age. Although greater sensitivity in some older individuals cannot be ruled out, no overall differences were observed in the safety and effectiveness of INLYTA between patients who were ≥65 years of age and younger.
Of the 434 patients randomized to INLYTA 5 mg twice daily administered in combination with avelumab 10 mg/kg in the JAVELIN Renal 101 trial, 38% were 65 years or older and 8% were 75 years or older. No overall difference in safety or efficacy was reported between patients who were ≥65 years of age and younger.
Of the 432 patients randomized to INLYTA 5 mg twice daily administered in combination with pembrolizumab 200 mg in the KEYNOTE-426 trial, 40% were 65 years or older. No overall difference in safety or efficacy was reported between patients who were ≥65 years of age and younger.
No dosage adjustment is required in elderly patients [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
In a dedicated hepatic impairment trial, compared to subjects with normal hepatic function, systemic exposure following a single dose of INLYTA was similar in subjects with baseline mild hepatic impairment (Child-Pugh class A) and higher in subjects with baseline moderate hepatic impairment (Child-Pugh class B).
No starting dose adjustment is required when administering INLYTA to patients with mild hepatic impairment (Child-Pugh class A). A starting dose decrease is recommended when administering INLYTA to patients with moderate hepatic impairment (Child-Pugh class B) [see Dosage and Administration (2.2), Warnings and Precautions (5.12), Clinical Pharmacology (12.3)].
INLYTA has not been studied in subjects with severe hepatic impairment (Child-Pugh class C).
8.7 Renal Impairment
No dedicated renal impairment trial for axitinib has been conducted. Based on the population pharmacokinetic analyses, no significant difference in axitinib clearance was observed in patients with pre-existing mild to severe renal impairment (15 mL/min ≤creatinine clearance [CLcr] <89 mL/min) [see Clinical Pharmacology (12.3)]. No starting dose adjustment is needed for patients with pre-existing mild to severe renal impairment. Caution should be used in patients with end-stage renal disease (CLcr <15 mL/min).
Overdosage
10 OVERDOSAGE
There is no specific treatment for INLYTA overdose.
In a controlled clinical study with INLYTA for the treatment of patients with RCC, 1 patient inadvertently received a dose of 20 mg twice daily for 4 days and experienced dizziness (Grade 1).
In a clinical dose finding study with INLYTA, subjects who received starting doses of 10 mg twice daily or 20 mg twice daily experienced adverse reactions which included hypertension, seizures associated with hypertension, and fatal hemoptysis.
In cases of suspected overdose, INLYTA should be withheld and supportive care instituted.
Description
11 DESCRIPTION
INLYTA (axitinib) is a kinase inhibitor. Axitinib has the chemical name N-methyl-2-[3-((E)-2-pyridin-2-yl-vinyl)-1H-indazol-6-ylsulfanyl]-benzamide. The molecular formula is C22H18N4OS and the molecular weight is 386.47 Daltons. The chemical structure is:
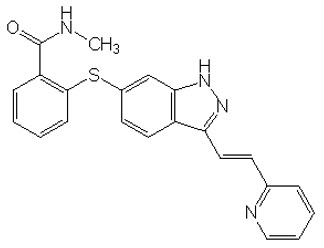
Axitinib is a white to light-yellow powder with a pKa of 4.8. The solubility of axitinib in aqueous media over the range pH 1.1 to pH 7.8 is in excess of 0.2 µg/mL. The partition coefficient (n-octanol/water) is 3.5.
INLYTA is supplied as red, film-coated tablets containing either 1 mg or 5 mg of axitinib together with microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, magnesium stearate, and Opadry II red 32K15441 as inactive ingredients. The Opadry II red 32K15441 film coating contains lactose monohydrate, HPMC 2910/Hypromellose 15cP, titanium dioxide, triacetin (glycerol triacetate), and red iron oxide.
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Axitinib has been shown to inhibit receptor tyrosine kinases including vascular endothelial growth factor receptors (VEGFR)-1, VEGFR-2, and VEGFR-3 at therapeutic plasma concentrations. These receptors are implicated in pathologic angiogenesis, tumor growth, and cancer progression. VEGF-mediated endothelial cell proliferation and survival were inhibited by axitinib in vitro and in mouse models. Axitinib was shown to inhibit tumor growth and phosphorylation of VEGFR-2 in tumor xenograft mouse models.
12.2 Pharmacodynamics
The effect of a single oral dose of INLYTA (5 mg) in the absence and presence of 400 mg ketoconazole on the QTc interval was evaluated in a randomized, single-blinded, two-way crossover study in 35 healthy subjects. No large changes in mean QTc interval (i.e., >20 ms) from placebo were detected up to 3 hours post-dose. However, small increases in mean QTc interval (i.e., <10 ms) cannot be ruled out.
12.3 Pharmacokinetics
The population pharmacokinetic analysis pooled data from 17 trials in healthy subjects and patients with cancer. A two-compartment disposition model with first-order absorption and lag-time adequately describes the axitinib concentration-time profile.
Absorption and Distribution
Following single oral 5-mg dose administration, the median Tmax ranged from 2.5 to 4.1 hours. Based on the plasma half-life, steady state is expected within 2 to 3 days of dosing. Dosing of axitinib at 5 mg twice daily resulted in approximately 1.4-fold accumulation compared to administration of a single dose. At steady state, axitinib exhibits approximately linear pharmacokinetics within the 1-mg to 20-mg dose range. The mean absolute bioavailability of axitinib after an oral 5 mg dose is 58%.
Compared to overnight fasting, administration of INLYTA with a moderate fat meal resulted in 10% lower AUC and a high fat, high-calorie meal resulted in 19% higher AUC. INLYTA can be administered with or without food [see Dosage and Administration (2.1)].
Axitinib is highly bound (>99%) to human plasma proteins with preferential binding to albumin and moderate binding to α1-acid glycoprotein. In patients with advanced RCC (n=20), at the 5 mg twice daily dose in the fed state, the geometric mean (CV%) Cmax and AUC0–24 were 27.8 (79%) ng/mL and 265 (77%) ng.h/mL, respectively. The geometric mean (CV%) clearance and apparent volume of distribution were 38 (80%) L/h and 160 (105%) L, respectively.
Metabolism and Elimination
The plasma half-life of INLYTA ranges from 2.5 to 6.1 hours. Axitinib is metabolized primarily in the liver by CYP3A4/5 and to a lesser extent by CYP1A2, CYP2C19, and UGT1A1. Following oral administration of a 5-mg radioactive dose of axitinib, approximately 41% of the radioactivity was recovered in feces and approximately 23% was recovered in urine. Unchanged axitinib, accounting for 12% of the dose, was the major component identified in feces. Unchanged axitinib was not detected in urine; the carboxylic acid and sulfoxide metabolites accounted for the majority of radioactivity in urine. In plasma, the N-glucuronide metabolite represented the predominant radioactive component (50% of circulating radioactivity) and unchanged axitinib and the sulfoxide metabolite each accounted for approximately 20% of the circulating radioactivity.
The sulfoxide and N-glucuronide metabolites show approximately ≥400-fold less in vitro potency against VEGFR-2 compared to axitinib.
Drug-Drug Interactions
Effects of Other Drugs on INLYTA
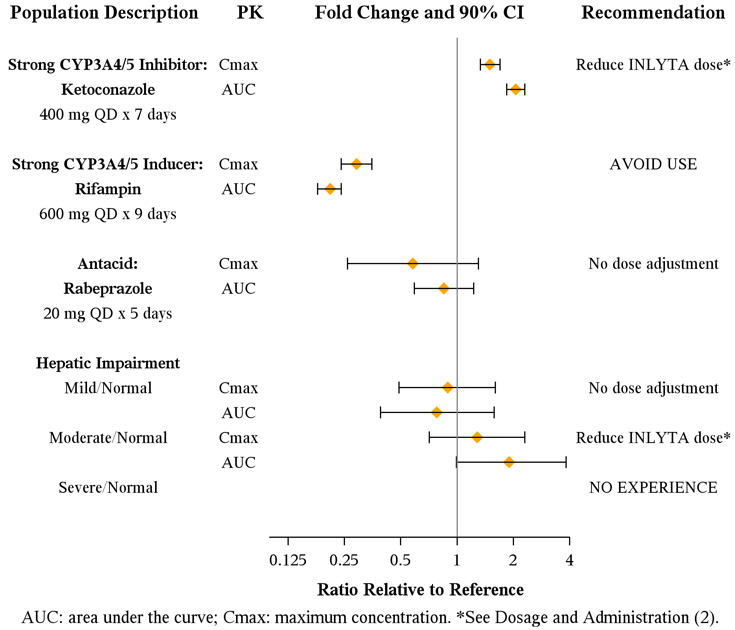
Axitinib is metabolized primarily in the liver by CYP3A4/5. Additionally, the aqueous solubility of axitinib is pH dependent, with higher pH resulting in lower solubility. The effects of a strong CYP3A4/5 inhibitor, a strong CYP3A4/5 inducer, and an antacid on the pharmacokinetics of axitinib are presented in Figure 1 [see Dosage and Administration (2.2) and Drug Interactions (7.1, 7.2)].
Figure 1. Impact of Co-administered Drugs and Hepatic Impairment on Axitinib Pharmacokinetics
Effects of INLYTA on Other Drugs
In vitro studies demonstrated that axitinib has the potential to inhibit CYP1A2 and CYP2C8. However, co-administration of axitinib with paclitaxel, a CYP2C8 substrate, did not increase plasma concentrations of paclitaxel in patients.
In vitro studies indicated that axitinib does not inhibit CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5, or UGT1A1 at therapeutic plasma concentrations. In vitro studies in human hepatocytes indicated that axitinib does not induce CYP1A1, CYP1A2, or CYP3A4/5.
Axitinib is an inhibitor of the efflux transporter P-glycoprotein (P-gp) in vitro. However, INLYTA is not expected to inhibit P-gp at therapeutic plasma concentrations.
Specific Populations
Patients with Hepatic Impairment
The effects of hepatic impairment on the pharmacokinetics of axitinib are presented in Figure 1 [see Dosage and Administration (2.2), Warnings and Precautions (5.12), Use in Specific Populations (8.6)].
Patients with Renal Impairment
Population pharmacokinetic analysis (based on pre-existing renal function) was carried out in 590 healthy volunteers and patients, including five with severe renal impairment (15 mL/min ≤CLcr <29 mL/min), 64 with moderate renal impairment (30 mL/min ≤CLcr <59 mL/min), and 139 with mild renal impairment (60 mL/min ≤CLcr <89 mL/min). Mild to severe renal impairment did not have meaningful effects on the pharmacokinetics of axitinib. Data from only one patient with end-stage renal disease are available [see Use in Specific Populations (8.7)].
Other Intrinsic Factors
Population pharmacokinetic analyses indicate that there are no clinically relevant effects of age, gender, race, body weight, body surface area, UGT1A1 genotype, or CYP2C19 genotype on the clearance of axitinib.
INLYTA in Combination with Avelumab
When INLYTA 5 mg was administered in combination with avelumab 10 mg/kg, the respective exposures of INLYTA and avelumab were comparable to the single agents. There was no evidence to suggest a clinically relevant change of avelumab clearance over time in patients with advanced RCC.
Nonclinical Toxicology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with axitinib.
Axitinib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay and was not clastogenic in the in vitro human lymphocyte chromosome aberration assay. Axitinib was genotoxic in the in vivo mouse bone marrow micronucleus assay.
INLYTA has the potential to impair reproductive function and fertility in humans. In repeat-dose toxicology studies, findings in the male reproductive tract were observed in the testes/epididymis (decreased organ weight, atrophy or degeneration, decreased numbers of germinal cells, hypospermia or abnormal sperm forms, reduced sperm density and count) at ≥15 mg/kg/dose administered orally twice daily in mice (approximately 7 times the systemic exposure (AUC) in patients at the recommended starting dose) and ≥1.5 mg/kg/dose administered orally twice daily in dogs (approximately 0.1 times the AUC in patients at the recommended starting dose). Findings in the female reproductive tract in mice and dogs included signs of delayed sexual maturity, reduced or absent corpora lutea, decreased uterine weights and uterine atrophy at ≥5 mg/kg/dose (approximately 1.5 or 0.3 times the AUC in patients at the recommended starting dose compared to mice and dogs, respectively).
In a fertility study in mice, axitinib did not affect mating or fertility rate when administered orally twice daily to males at any dose tested up to 50 mg/kg/dose following at least 70 days of administration (approximately 57 times the AUC in patients at the recommended starting dose). In female mice, reduced fertility and embryonic viability were observed at all doses tested (≥15 mg/kg/dose administered orally twice daily) following at least 15 days of treatment with axitinib (approximately 10 times the AUC in patients at the recommended starting dose).
Clinical Studies
14 CLINICAL STUDIES
14.1 First-Line Advanced RCC
INLYTA in Combination with Avelumab
The efficacy and safety of INLYTA in combination with avelumab was demonstrated in the JAVELIN Renal 101 trial (NCT02684006), a randomized, multicenter, open-label, study of INLYTA in combination with avelumab in 886 patients with untreated advanced RCC regardless of tumor PD-L1 expression [intent-to-treat (ITT) population]. Patients with autoimmune disease or conditions requiring systemic immunosuppression were excluded.
Randomization was stratified according to Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) (0 vs. 1) and region (United States vs. Canada/Western Europe vs. the rest of the world). Patients were randomized (1:1) to one of the following treatment arms:
- INLYTA 5 mg twice daily orally was given in combination with avelumab 10 mg/kg intravenous infusion every 2 weeks (N=442). Patients who tolerated INLYTA 5 mg twice daily without Grade 2 or greater INLYTA-related adverse events for 2 consecutive weeks could increase to 7 mg and then subsequently to 10 mg twice daily. INLYTA could be interrupted or reduced to 3 mg twice daily and subsequently to 2 mg twice daily to manage toxicity.
- Sunitinib 50 mg once daily orally for 4 weeks followed by 2 weeks off (N=444) until radiographic or clinical progression or unacceptable toxicity.
Treatment with INLYTA and avelumab continued until RECIST v1.1-defined progression of disease by Blinded Independent Central Review (BICR) assessment or unacceptable toxicity. Administration of INLYTA and avelumab was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumor status was performed at baseline, after randomization at 6 weeks, then every 6 weeks thereafter up to 18 months after randomization, and every 12 weeks thereafter until documented confirmed disease progression by BICR.
Baseline characteristics were a median age of 61 years (range: 27 to 88), 38% of patients were 65 years or older, 75% were male, 75% were White, and the ECOG PS was 0 (63%) or 1 (37%), respectively. Patient distribution by International Metastatic Renal Cell Carcinoma Database (IMDC) risk groups was 21% favorable, 62% intermediate, and 16% poor.
The major efficacy outcome measures were progression-free survival (PFS), as assessed by an BICR using RECIST v1.1 and overall survival (OS) in patients with PD-L1-positive tumors using a clinical trial assay (PD-L1 expression level ≥1%). Since PFS was statistically significant in patients with PD-L1-positive tumors [HR 0.61 (95% CI: 0.48, 0.79)], it was then tested in the ITT population and a statistically significant improvement in PFS in the ITT population was also demonstrated.
With a median overall survival follow-up of 19 months, overall survival data were immature with 27% deaths in the ITT population.
Efficacy results are presented in Table 10 and Figure 2.
| Efficacy Endpoints (Based on BICR Assessment) | INLYTA plus avelumab (N=422) | Sunitinib (N=444) |
|---|---|---|
| BICR: Blinded Independent Central Review; CI: Confidence interval; NE: Not estimable. | ||
| ||
| Progression-Free Survival (PFS) | ||
| Events (%) | 180 (41) | 216 (49) |
| Median in Months (95% CI) | 13.8 (11.1, NE) | 8.4 (6.9, 11.1) |
| Hazard ratio (95% CI) | 0.69 (0.56, 0.84) | |
| 2-sided p-value* | 0.0002 | |
| Confirmed Objective Response Rate (ORR) | ||
| Objective Response Rate n (%) | 227 (51.4) | 114 (25.7) |
| (95% CI) | (46.6, 56.1) | (21.7, 30.0) |
| Complete Response (CR) n (%) | 15 (3.4) | 8 (1.8) |
| Partial Response (PR) n (%) | 212 (48) | 106 (24) |
Figure 2. K-M Estimates for PFS Based on BICR Assessment - ITT
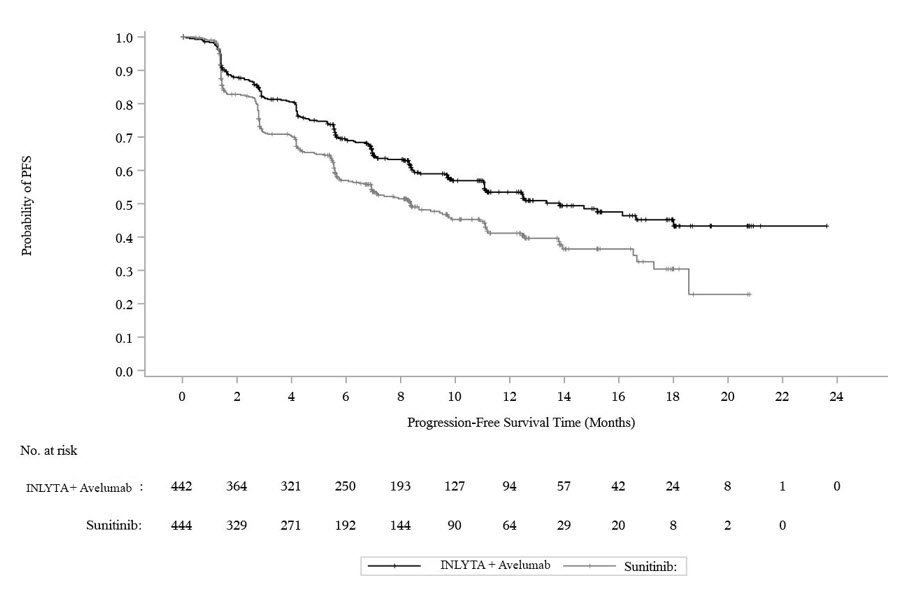
INLYTA in Combination with Pembrolizumab
The efficacy of INLYTA in combination with pembrolizumab was investigated in KEYNOTE-426 (NCT02853331), a randomized, multicenter, open-label trial conducted in 861 patients who had not received systemic therapy for advanced RCC. Patients were enrolled regardless of PD-L1 tumor expression status. Patients with active autoimmune disease requiring systemic immunosuppression within the last 2 years were ineligible. Randomization was stratified by International Metastatic RCC Database Consortium (IMDC) risk categories (favorable versus intermediate versus poor) and geographic region (North America versus Western Europe versus "Rest of the World").
Patients were randomized (1:1) to one of the following treatment arms:
- INLYTA 5 mg orally, twice daily in combination with pembrolizumab 200 mg intravenously every 3 weeks up to 24 months. Patients who tolerated INLYTA 5 mg twice daily for 2 consecutive cycles (6 weeks) could increase to 7 mg and then subsequently to 10 mg twice daily. INLYTA could be interrupted or reduced to 3 mg twice daily and subsequently to 2 mg twice daily to manage toxicity.
- Sunitinib 50 mg orally, once daily for 4 weeks and then off treatment for 2 weeks.
Treatment with INLYTA and pembrolizumab continued until RECIST v1.1-defined progression of disease or unacceptable toxicity. Administration of INLYTA and pembrolizumab was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumor status was performed at baseline, after randomization at Week 12, then every 6 weeks thereafter until Week 54, and then every 12 weeks thereafter.
The study population characteristics were: median age of 62 years (range: 26 to 90); 38% age 65 or older; 73% male; 79% White and 16% Asian; 20% and 80% of patients had a baseline KPS of 70 to 80 and 90 to 100, respectively; and patient distribution by IMDC risk categories was 31% favorable, 56% intermediate and 13% poor.
The main efficacy outcome measures were OS and PFS as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ. Additional efficacy outcome measures included ORR, as assessed by BICR. A statistically significant improvement in OS was demonstrated at the first pre-specified interim analysis in patients randomized to INLYTA in combination with pembrolizumab compared with sunitinib. The trial also demonstrated statistically significant improvements in PFS and ORR.
An updated OS analysis was conducted when 418 deaths were observed based on the planned number of deaths for the pre-specified final analysis. Table 11 and Figure 3 summarize the efficacy results for KEYNOTE-426.
| Endpoint | INLYTA and Pembrolizumab N=432 | Sunitinib N=429 |
|---|---|---|
| CI: confidence interval; NR: not reached; ORR: objective response rate; OS: overall survival; PFS: progression-free survival. | ||
| ||
| OS | ||
| Number of patients with event (%) | 59 (14%) | 97 (23%) |
| Median in months (95% CI) | NR (NR, NR) | NR (NR, NR) |
| Hazard ratio* (95% CI) | 0.53 (0.38, 0.74) | |
| p-Value † | <0.0001 ‡ | |
| 12-month OS rate | 90% (86, 92) | 78% (74, 82) |
| Updated OS | ||
| Number of patients with event (%) | 193 (45%) | 225 (52%) |
| Median in months (95% CI) | 45.7 (43.6, NR) | 40.1 (34.3, 44.2) |
| Hazard ratio* (95% CI) | 0.73 (0.60, 0.88) | |
| PFS | ||
| Number of patients with event (%) | 183 (42%) | 213 (50%) |
| Median in months (95% CI) | 15.1 (12.6, 17.7) | 11.0 (8.7, 12.5) |
| Hazard ratio* (95% CI) | 0.69 (0.56, 0.84) | |
| p-Value † | 0.0001§ | |
| ORR | ||
| Overall confirmed response rate (95% CI) | 59% (54, 64) | 36% (31, 40) |
| Complete response rate | 6% | 2% |
| Partial response rate | 53% | 34% |
| p-Value¶ | <0.0001 | |
Figure 3. Kaplan-Meier Curve for Overall Survival in KEYNOTE-426
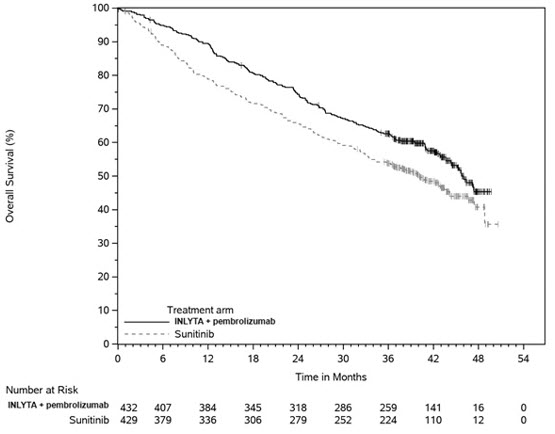
In an exploratory analysis, the updated analysis of OS in patients with IMDC favorable, intermediate, intermediate/poor, and poor risk demonstrated a HR of 1.17 (95% CI: 0.76, 1.80), 0.67 (95% CI: 0.52, 0.86), 0.64 (95% CI: 0.52, 0.80), and 0.51 (95% CI: 0.32, 0.81), respectively.
14.2 Second-Line Advanced RCC
The safety and efficacy of INLYTA were evaluated in a randomized, open-label, multicenter Phase 3 study. Patients (N=723) with advanced RCC whose disease had progressed on or after treatment with 1 prior systemic therapy, including sunitinib-, bevacizumab-, temsirolimus-, or cytokine-containing regimens were randomized (1:1) to receive INLYTA (N=361) or sorafenib (N=362). Progression-free survival (PFS) was assessed by a blinded independent central review committee. Other endpoints included objective response rate (ORR) and overall survival (OS).
Of the patients enrolled in this study, 389 patients (54%) had received 1 prior sunitinib-based therapy, 251 patients (35%) had received 1 prior cytokine-based therapy (interleukin-2 or interferon-alfa), 59 patients (8%) had received 1 prior bevacizumab-based therapy, and 24 patients (3%) had received 1 prior temsirolimus-based therapy. The baseline demographic and disease characteristics were similar between the INLYTA and sorafenib groups with regard to age (median 61 years), gender (72% male), race (75% white, 21% Asian), Eastern Cooperative Oncology Group (ECOG) performance status (55% 0, 45% 1), and histology (99% clear cell).
There was a statistically significant advantage for INLYTA over sorafenib for the endpoint of PFS (see Table 12 and Figure 4). There was no statistically significant difference between the arms in OS.
| Endpoint/Study Population | INLYTA | Sorafenib | HR (95% CI) | P-value |
|---|---|---|---|---|
| CI: Confidence interval; HR: Hazard ratio (INLYTA/sorafenib); ITT: Intent-to-treat; ORR: Objective response rate; NS: Not significant; OS: Overall survival; PFS: Progression-free survival | ||||
| ||||
| Overall ITT | N= 361 | N = 362 | ||
| Median PFS*,† in months (95% CI) | 6.7 (6.3, 8.6) | 4.7 (4.6, 5.6) | 0.67 (0.54, 0.81) | <0.0001‡ |
| Median OS in months (95% CI) | 20.1 (16.7, 23.4) | 19.2 (17.5, 22.3) | 0.97 (0.80, 1.17) | NS |
| ORR % (95% CI) | 19.4 (15.4, 23.9) | 9.4 (6.6, 12.9) | 2.06§ (1.41, 3.00) | -¶ |
| PFS by prior treatment | ||||
| Sunitinib-refractory subgroup | N=194 | N=195 | ||
| Median, months (95% CI) | 4.8 (4.5, 6.4) | 3.4 (2.8, 4.7) | 0.74 (0.57, 0.96) | -¶ |
| Cytokine-refractory subgroup | N=126 | N=125 | ||
| Median, months (95% CI) | 12.1 (10.1, 13.9) | 6.5 (6.3, 8.3) | 0.46 (0.32, 0.68) | -¶ |
Figure 4. Kaplan-Meier Curve for Progression-Free Survival by Independent Assessment (Intent-to-Treat Population)
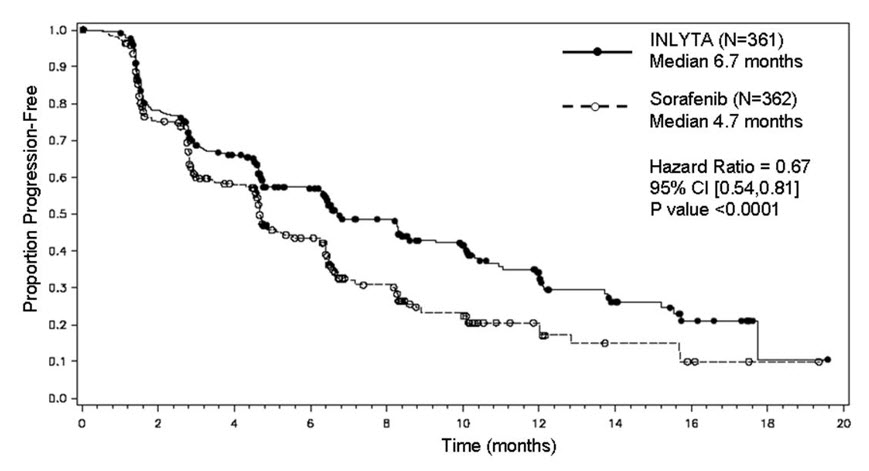
How Supplied/Storage and Handling
16 HOW SUPPLIED/STORAGE AND HANDLING
INLYTA tablets are supplied as follows:
- 1 mg tablets are red film-coated, oval tablets debossed with "Pfizer" on one side and "1 XNB" on the other; available in bottles of 180: NDC 0069-0145-01.
- 5 mg tablets are red film-coated, triangular tablets debossed with "Pfizer" on one side and "5 XNB" on the other; available in bottles of 60: NDC 0069-0151-11.
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Medication Guide
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypertension
Advise patients that hypertension may develop during INLYTA treatment and that blood pressure should be monitored regularly during treatment [see Warnings and Precautions (5.1)].
Arterial/Venous Thromboembolic Events
Advise patients that arterial and venous thromboembolic events have been observed during INLYTA treatment and to inform their doctor if they experience symptoms suggestive of thromboembolic events [see Warnings and Precautions (5.2, 5.3)].
Hemorrhage
Advise patients that INLYTA may increase the risk of bleeding and to promptly inform their doctor of any bleeding episodes [see Warnings and Precautions (5.4)].
Cardiac Failure
Advise patients that cardiac failure may develop during INLYTA treatment and that signs or symptoms of cardiac failure should be regularly monitored for during treatment [see Warnings and Precautions (5.5)].
Gastrointestinal Disorders
Advise patients that gastrointestinal disorders such as diarrhea, nausea, vomiting, and constipation may develop during INLYTA treatment and to seek immediate medical attention if they experience persistent or severe abdominal pain because cases of gastrointestinal perforation and fistula have been reported in patients taking INLYTA [see Warnings and Precautions (5.6) and Adverse Reactions (6.1)].
Abnormal Thyroid Function
Advise patients that abnormal thyroid function may develop during INLYTA treatment and to inform their doctor if symptoms of abnormal thyroid function occur [see Warnings and Precautions (5.7)].
Impaired Wound Healing
Advise patients that INLYTA may impair wound healing. Advise patients to inform their healthcare provider of any planned surgical procedure [see Warnings and Precautions (5.8)].
Reversible Posterior Leukoencephalopathy Syndrome
Advise patients to inform their doctor if they have worsening of neurological function consistent with RPLS (headache, seizure, lethargy, confusion, blindness and other visual and neurologic disturbances) [see Warnings and Precautions (5.9)].
Hepatotoxicity
Inform patients of the signs and symptoms of hepatotoxicity. Advise patients to contact their healthcare provider immediately for signs or symptoms of hepatotoxicity [see Warnings and Precautions (5.11)].
Major Adverse Cardiovascular Events
Advise patients receiving INLYTA in combination with avelumab to contact their healthcare provider immediately for signs or symptoms of cardiovascular events including but not limited to new or worsening chest discomfort, dyspnea, or peripheral edema [see Warnings and Precautions (5.13)].
Embryo-Fetal Toxicity
Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy [see Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with INLYTA and for 1 week after the last dose.
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 1 week following the last dose [see Warnings and Precautions (5.14) and Use in Specific Populations (8.3)].
When INLYTA is used in combination with avelumab or pembrolizumab, refer to the full prescribing information of avelumab or pembrolizumab for pregnancy and contraception information.
Lactation
Advise patients not to breastfeed while taking INLYTA and for 2 weeks after receiving the last dose [see Use in Specific Populations (8.2)].
When INLYTA is used in combination with avelumab or pembrolizumab, refer to the full prescribing information of avelumab or pembrolizumab for lactation information.
Infertility
Advise males and females of reproductive potential that INLYTA may impair fertility [see Use in Specific Populations (8.3)].
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: September 2022 | ||
| PATIENT INFORMATION INLYTA (in-ly-ta) (axitinib) tablets | |||
| Important information: If your healthcare provider prescribes INLYTA for you to be taken with avelumab or pembrolizumab, also read the Medication Guide for avelumab or pembrolizumab. | |||
| What is INLYTA? INLYTA is a prescription medicine used to treat kidney cancer that has spread or cannot be removed by surgery (advanced renal cell carcinoma or RCC):
| |||
Before taking INLYTA, tell your healthcare provider about all of your medical conditions, including if you:
Talk with your healthcare provider before you start taking any new medicine. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. | |||
How should I take INLYTA?
| |||
What should I avoid while taking INLYTA?
| |||
| What are the possible side effects of INLYTA? INLYTA may cause serious side effects, including:
| |||
|
| ||
| |||
|
| ||
| |||
| |||
|
| ||
| |||
| |||
|
| ||
| |||
| |||
|
| ||
| |||
|
| ||
| |||
|
| ||
| The most common side effects of INLYTA with avelumab include: | |||
|
| ||
| The most common side effects of INLYTA with pembrolizumab include: | |||
|
| ||
| The most common side effects of INLYTA when used alone include: | |||
|
| ||
| INLYTA may cause fertility problems in males and females, which may affect your ability to have a child. Talk to your healthcare provider if this is a concern for you. These are not all of the possible side effects of INLYTA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
| How should I store INLYTA? Store INLYTA at room temperature between 68°F to 77°F (20°C to 25°C). Keep INLYTA and all medicines out of the reach of children. | |||
| General information about the safe and effective use of INLYTA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use INLYTA for a condition for which it was not prescribed. Do not give INLYTA to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about INLYTA that is written for health professionals. | |||
| What are the ingredients in INLYTA? Active ingredient: axitinib Inactive ingredients: microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, magnesium stearate, and Opadry II red 32K15441. The Opadry II red 32K15441 film coating contains: lactose monohydrate, HPMC 2910/Hypromellose 15cP, titanium dioxide, triacetin (glycerol triacetate), and red iron oxide. | |||
| LAB-0439-7.0 | ||
| For more information, go to www.inlyta.com or call 8770744-5675 This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com. | |||
Other
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information. 9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.
