DEPO-SUBQ PROVERA 104® Clinical Pharmacology
(medroxyprogesterone acetate)
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Depo-subQ provera 104 inhibits the secretion of gonadotropins, which primarily prevents follicular maturation and ovulation and causes thickening of cervical mucus. These actions contribute to its contraceptive effect.
Suppression of serum estradiol concentrations is likely to be responsible for the therapeutic effect on endometriosis-associated pain.
12.2 Pharmacodynamics
The following laboratory tests are expected to be affected by progestins including depo-subQ provera 104:
- Plasma and urinary steroid levels are decreased (e.g., progesterone, estradiol, pregnanediol, testosterone, cortisol).
- Gonadotropin levels are decreased.
- Sex-hormone-binding-globulin concentrations are decreased.
- Histology specimens may demonstrate changes consistent with progestin effects.
The following laboratory tests may be affected by depo-subQ provera 104, however the clinical significance is unknown:
- Protein-bound iodine and butanol extractable protein-bound iodine may increase.
- T3-uptake values may decrease.
- Coagulation test values for prothrombin (Factor II), and Factors VII, VIII, IX, and X may increase.
- Sulfobromophthalein and other liver function test values may be increased.
- The effects of medroxyprogesterone acetate on lipid metabolism are inconsistent. Both increases and decreases in total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol have been observed in studies.
12.3 Pharmacokinetics
The pharmacokinetic parameters of MPA following a single subcutaneous injection of depo-subQ provera 104 in healthy women (n=42) are shown in Table 2 and Figure P.
| Cmax (ng/mL) | Tmax (day) | C91 (ng/mL) | AUC0–91 (ng∙day/mL) | AUC0–∞ (ng∙day/mL) | t½ (day) | |
|---|---|---|---|---|---|---|
| Abbreviations: Cmax=peak serum concentration; Tmax=time when Cmax is observed; C91=serum concentration at 91 days; AUC0–91 and AUC0–∞=area under the concentration-time curve over 91 days or infinity, respectively; t½=terminal half-life. | ||||||
| Mean | 1.56 | 8.8 | 0.402 | 66.98 | 92.84 | 43 |
| Min | 0.53 | 2.0 | 0.133 | 20.63 | 31.36 | 16 |
| Max | 3.08 | 80.0 | 0.733 | 139.79 | 162.29 | 114 |
Following subcutaneous administration of single depo-subQ provera 104 doses ranging from 50 to 150 mg (0.48 and 1.4 times the recommended dose, respectively), the AUC and Cmin (Day 91) increased with higher doses, but there was considerable overlap across dose levels. Serum MPA concentrations at Day 91 increased in a dose proportional manner but Cmax did not appear to increase proportionally with increasing dose. The AUC data were suggestive of dose linearity.
Absorption
Following a single subcutaneous injection of depo-subQ provera 104 in healthy women, serum MPA concentrations reached ≥0.2 ng/mL within 24 hours. The mean Tmax was attained approximately 1 week after injection.
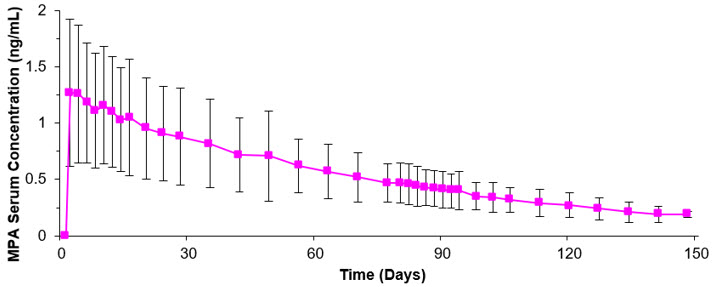 |
In a study to assess accumulation and the achievement of steady state following multiple subcutaneous administrations, trough concentrations of MPA were determined after 6, 12, and 24 months, and in a subset of 8 subjects, bi-weekly concentrations were determined within one dosing interval in the second year of administration. The mean (SD) MPA trough concentrations were 0.67 (0.36) ng/mL (n=157), 0.79 (0.36) ng/mL (n=144), and 0.87 (0.33) ng/mL (n=106) at 6, 12, and 24 months, respectively.
Depo-subQ provera 104 was administered subcutaneously into the anterior thigh or the abdomen to evaluate effects of injection site location on the MPA concentration-time profile. MPA trough concentrations (Cmin; Day 91) were similar for the two injection site locations.
Distribution
Plasma protein binding of MPA averages 86%. MPA binding occurs primarily to serum albumin. No binding of MPA occurs with sex-hormone-binding globulin (SHBG).
Elimination
Metabolism
MPA is extensively metabolized in the liver by P450 enzymes. Its metabolism primarily involves ring A and/or side-chain reduction, loss of the acetyl group, hydroxylation in the 2-, 6-, and 21-positions or a combination of these positions, resulting in more than 10 metabolites.
Excretion
Residual MPA concentrations at the end of the first dosing interval (12 to 14 weeks) of depo-subQ provera 104 were generally below 0.5 ng/mL, consistent with its apparent terminal half-life of ~40 days after subcutaneous administration. Most MPA metabolites were excreted in the urine as glucuronide conjugates with only small amounts excreted as sulfates.
Specific Populations
Racial Groups
There were no significant differences in the pharmacokinetics and/or pharmacodynamics of MPA after subcutaneous administration of depo-subQ provera 104 in African-American, Caucasian, and Asian women.
Effect of Body Weight
Although total MPA exposure was lower in obese women, no dosage adjustment of depo-subQ provera 104 is necessary based on body weight. The effect of body weight on the pharmacokinetics of MPA following a single dose was assessed in a subset of women [n=42, body mass index (BMI) ranged from 18.2 to 46.7 kg/m2]. The AUC0–91 values for MPA were 71.6, 67.9, and 46.3 ng∙day/mL in women with BMI categories of ≤28 kg/m2, >28–38 kg/m2, and >38 kg/m2, respectively. The mean MPA Cmax was 1.74 ng/mL in women with BMI ≤28 kg/m2, 1.53 ng/mL in women with BMI >28–38 kg/m2, and 1.02 ng/mL in women with BMI >38 kg/m2, respectively. The MPA trough (Cmin) concentrations had a tendency to be lower in women with BMI >38 kg/m2.
Find DEPO-SUBQ PROVERA 104® medical information:
Find DEPO-SUBQ PROVERA 104® medical information:
DEPO-SUBQ PROVERA 104® Quick Finder
Health Professional Information
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Depo-subQ provera 104 inhibits the secretion of gonadotropins, which primarily prevents follicular maturation and ovulation and causes thickening of cervical mucus. These actions contribute to its contraceptive effect.
Suppression of serum estradiol concentrations is likely to be responsible for the therapeutic effect on endometriosis-associated pain.
12.2 Pharmacodynamics
The following laboratory tests are expected to be affected by progestins including depo-subQ provera 104:
- Plasma and urinary steroid levels are decreased (e.g., progesterone, estradiol, pregnanediol, testosterone, cortisol).
- Gonadotropin levels are decreased.
- Sex-hormone-binding-globulin concentrations are decreased.
- Histology specimens may demonstrate changes consistent with progestin effects.
The following laboratory tests may be affected by depo-subQ provera 104, however the clinical significance is unknown:
- Protein-bound iodine and butanol extractable protein-bound iodine may increase.
- T3-uptake values may decrease.
- Coagulation test values for prothrombin (Factor II), and Factors VII, VIII, IX, and X may increase.
- Sulfobromophthalein and other liver function test values may be increased.
- The effects of medroxyprogesterone acetate on lipid metabolism are inconsistent. Both increases and decreases in total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol have been observed in studies.
12.3 Pharmacokinetics
The pharmacokinetic parameters of MPA following a single subcutaneous injection of depo-subQ provera 104 in healthy women (n=42) are shown in Table 2 and Figure P.
| Cmax (ng/mL) | Tmax (day) | C91 (ng/mL) | AUC0–91 (ng∙day/mL) | AUC0–∞ (ng∙day/mL) | t½ (day) | |
|---|---|---|---|---|---|---|
| Abbreviations: Cmax=peak serum concentration; Tmax=time when Cmax is observed; C91=serum concentration at 91 days; AUC0–91 and AUC0–∞=area under the concentration-time curve over 91 days or infinity, respectively; t½=terminal half-life. | ||||||
| Mean | 1.56 | 8.8 | 0.402 | 66.98 | 92.84 | 43 |
| Min | 0.53 | 2.0 | 0.133 | 20.63 | 31.36 | 16 |
| Max | 3.08 | 80.0 | 0.733 | 139.79 | 162.29 | 114 |
Following subcutaneous administration of single depo-subQ provera 104 doses ranging from 50 to 150 mg (0.48 and 1.4 times the recommended dose, respectively), the AUC and Cmin (Day 91) increased with higher doses, but there was considerable overlap across dose levels. Serum MPA concentrations at Day 91 increased in a dose proportional manner but Cmax did not appear to increase proportionally with increasing dose. The AUC data were suggestive of dose linearity.
Absorption
Following a single subcutaneous injection of depo-subQ provera 104 in healthy women, serum MPA concentrations reached ≥0.2 ng/mL within 24 hours. The mean Tmax was attained approximately 1 week after injection.
 |
In a study to assess accumulation and the achievement of steady state following multiple subcutaneous administrations, trough concentrations of MPA were determined after 6, 12, and 24 months, and in a subset of 8 subjects, bi-weekly concentrations were determined within one dosing interval in the second year of administration. The mean (SD) MPA trough concentrations were 0.67 (0.36) ng/mL (n=157), 0.79 (0.36) ng/mL (n=144), and 0.87 (0.33) ng/mL (n=106) at 6, 12, and 24 months, respectively.
Depo-subQ provera 104 was administered subcutaneously into the anterior thigh or the abdomen to evaluate effects of injection site location on the MPA concentration-time profile. MPA trough concentrations (Cmin; Day 91) were similar for the two injection site locations.
Distribution
Plasma protein binding of MPA averages 86%. MPA binding occurs primarily to serum albumin. No binding of MPA occurs with sex-hormone-binding globulin (SHBG).
Elimination
Metabolism
MPA is extensively metabolized in the liver by P450 enzymes. Its metabolism primarily involves ring A and/or side-chain reduction, loss of the acetyl group, hydroxylation in the 2-, 6-, and 21-positions or a combination of these positions, resulting in more than 10 metabolites.
Excretion
Residual MPA concentrations at the end of the first dosing interval (12 to 14 weeks) of depo-subQ provera 104 were generally below 0.5 ng/mL, consistent with its apparent terminal half-life of ~40 days after subcutaneous administration. Most MPA metabolites were excreted in the urine as glucuronide conjugates with only small amounts excreted as sulfates.
Specific Populations
Racial Groups
There were no significant differences in the pharmacokinetics and/or pharmacodynamics of MPA after subcutaneous administration of depo-subQ provera 104 in African-American, Caucasian, and Asian women.
Effect of Body Weight
Although total MPA exposure was lower in obese women, no dosage adjustment of depo-subQ provera 104 is necessary based on body weight. The effect of body weight on the pharmacokinetics of MPA following a single dose was assessed in a subset of women [n=42, body mass index (BMI) ranged from 18.2 to 46.7 kg/m2]. The AUC0–91 values for MPA were 71.6, 67.9, and 46.3 ng∙day/mL in women with BMI categories of ≤28 kg/m2, >28–38 kg/m2, and >38 kg/m2, respectively. The mean MPA Cmax was 1.74 ng/mL in women with BMI ≤28 kg/m2, 1.53 ng/mL in women with BMI >28–38 kg/m2, and 1.02 ng/mL in women with BMI >38 kg/m2, respectively. The MPA trough (Cmin) concentrations had a tendency to be lower in women with BMI >38 kg/m2.
Health Professional Information
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Depo-subQ provera 104 inhibits the secretion of gonadotropins, which primarily prevents follicular maturation and ovulation and causes thickening of cervical mucus. These actions contribute to its contraceptive effect.
Suppression of serum estradiol concentrations is likely to be responsible for the therapeutic effect on endometriosis-associated pain.
12.2 Pharmacodynamics
The following laboratory tests are expected to be affected by progestins including depo-subQ provera 104:
- Plasma and urinary steroid levels are decreased (e.g., progesterone, estradiol, pregnanediol, testosterone, cortisol).
- Gonadotropin levels are decreased.
- Sex-hormone-binding-globulin concentrations are decreased.
- Histology specimens may demonstrate changes consistent with progestin effects.
The following laboratory tests may be affected by depo-subQ provera 104, however the clinical significance is unknown:
- Protein-bound iodine and butanol extractable protein-bound iodine may increase.
- T3-uptake values may decrease.
- Coagulation test values for prothrombin (Factor II), and Factors VII, VIII, IX, and X may increase.
- Sulfobromophthalein and other liver function test values may be increased.
- The effects of medroxyprogesterone acetate on lipid metabolism are inconsistent. Both increases and decreases in total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol have been observed in studies.
12.3 Pharmacokinetics
The pharmacokinetic parameters of MPA following a single subcutaneous injection of depo-subQ provera 104 in healthy women (n=42) are shown in Table 2 and Figure P.
| Cmax (ng/mL) | Tmax (day) | C91 (ng/mL) | AUC0–91 (ng∙day/mL) | AUC0–∞ (ng∙day/mL) | t½ (day) | |
|---|---|---|---|---|---|---|
| Abbreviations: Cmax=peak serum concentration; Tmax=time when Cmax is observed; C91=serum concentration at 91 days; AUC0–91 and AUC0–∞=area under the concentration-time curve over 91 days or infinity, respectively; t½=terminal half-life. | ||||||
| Mean | 1.56 | 8.8 | 0.402 | 66.98 | 92.84 | 43 |
| Min | 0.53 | 2.0 | 0.133 | 20.63 | 31.36 | 16 |
| Max | 3.08 | 80.0 | 0.733 | 139.79 | 162.29 | 114 |
Following subcutaneous administration of single depo-subQ provera 104 doses ranging from 50 to 150 mg (0.48 and 1.4 times the recommended dose, respectively), the AUC and Cmin (Day 91) increased with higher doses, but there was considerable overlap across dose levels. Serum MPA concentrations at Day 91 increased in a dose proportional manner but Cmax did not appear to increase proportionally with increasing dose. The AUC data were suggestive of dose linearity.
Absorption
Following a single subcutaneous injection of depo-subQ provera 104 in healthy women, serum MPA concentrations reached ≥0.2 ng/mL within 24 hours. The mean Tmax was attained approximately 1 week after injection.
 |
In a study to assess accumulation and the achievement of steady state following multiple subcutaneous administrations, trough concentrations of MPA were determined after 6, 12, and 24 months, and in a subset of 8 subjects, bi-weekly concentrations were determined within one dosing interval in the second year of administration. The mean (SD) MPA trough concentrations were 0.67 (0.36) ng/mL (n=157), 0.79 (0.36) ng/mL (n=144), and 0.87 (0.33) ng/mL (n=106) at 6, 12, and 24 months, respectively.
Depo-subQ provera 104 was administered subcutaneously into the anterior thigh or the abdomen to evaluate effects of injection site location on the MPA concentration-time profile. MPA trough concentrations (Cmin; Day 91) were similar for the two injection site locations.
Distribution
Plasma protein binding of MPA averages 86%. MPA binding occurs primarily to serum albumin. No binding of MPA occurs with sex-hormone-binding globulin (SHBG).
Elimination
Metabolism
MPA is extensively metabolized in the liver by P450 enzymes. Its metabolism primarily involves ring A and/or side-chain reduction, loss of the acetyl group, hydroxylation in the 2-, 6-, and 21-positions or a combination of these positions, resulting in more than 10 metabolites.
Excretion
Residual MPA concentrations at the end of the first dosing interval (12 to 14 weeks) of depo-subQ provera 104 were generally below 0.5 ng/mL, consistent with its apparent terminal half-life of ~40 days after subcutaneous administration. Most MPA metabolites were excreted in the urine as glucuronide conjugates with only small amounts excreted as sulfates.
Specific Populations
Racial Groups
There were no significant differences in the pharmacokinetics and/or pharmacodynamics of MPA after subcutaneous administration of depo-subQ provera 104 in African-American, Caucasian, and Asian women.
Effect of Body Weight
Although total MPA exposure was lower in obese women, no dosage adjustment of depo-subQ provera 104 is necessary based on body weight. The effect of body weight on the pharmacokinetics of MPA following a single dose was assessed in a subset of women [n=42, body mass index (BMI) ranged from 18.2 to 46.7 kg/m2]. The AUC0–91 values for MPA were 71.6, 67.9, and 46.3 ng∙day/mL in women with BMI categories of ≤28 kg/m2, >28–38 kg/m2, and >38 kg/m2, respectively. The mean MPA Cmax was 1.74 ng/mL in women with BMI ≤28 kg/m2, 1.53 ng/mL in women with BMI >28–38 kg/m2, and 1.02 ng/mL in women with BMI >38 kg/m2, respectively. The MPA trough (Cmin) concentrations had a tendency to be lower in women with BMI >38 kg/m2.
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information.9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.