BOSULIF®
(bosutinib)
Find BOSULIF® medical information:
Find BOSULIF® medical information:
BOSULIF® Quick Finder
What is BOSULIF?
What is BOSULIF?
- •
- adults and children 1 year of age and older who have a certain type of leukemia called chronic phase (CP) Philadelphia chromosome-positive chronic myelogenous leukemia (Ph+ CML) who are newly-diagnosed or who no longer benefit from or did not tolerate other treatment.
- •
- adults with accelerated phase (AP), or blast phase (BP) Ph+ CML who can no longer benefit from or did not tolerate other treatment.
It is not known if BOSULIF is safe and effective in children less than 1 year of age with CP Ph+ CML who are newly‑diagnosed or who no longer benefit from or did not tolerate other treatment or in children with AP Ph+ CML or BP Ph+ CML.
Do not take BOSULIF if you are allergic to bosutinib or any of the ingredients in BOSULIF. See the end of this leaflet for a complete list of ingredients of BOSULIF.
Before taking BOSULIF, tell your doctor about all of your medical conditions, including if you:
- •
- have liver problems
- •
- have heart problems
- •
- have kidney problems
- •
- have high blood pressure
- •
- have diabetes
- •
- are pregnant or plan to become pregnant. BOSULIF can harm your unborn baby. Females who are able to become pregnant should have a pregnancy test before starting treatment with BOSULIF. Tell your doctor right away if you become pregnant during treatment with BOSULIF.
- o
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with BOSULIF and for 2 weeks after the last dose. Talk to your doctor about birth control methods that may be right for you.
- •
- are breastfeeding or plan to breastfeed. It is not known if BOSULIF passes into your breast milk or if it can harm your baby. Do not breastfeed during treatment with BOSULIF and for 2 weeks after the last dose.
Tell your doctor about all the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements. When taken together, BOSULIF and certain other medicines can affect each other.
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist when you get a new medicine.
How should I take BOSULIF?
How should I take BOSULIF?
- •
- Take BOSULIF exactly as prescribed by your doctor.
- •
- Do not change your dose or stop taking BOSULIF without first talking with your doctor.
- •
- If your child takes BOSULIF, your healthcare provider will change the dose as your child grows.
- •
- Take BOSULIF with food.
- •
- Swallow BOSULIF tablets whole. Do not crush, break, chew or cut BOSULIF tablets. Do not touch or handle crushed or broken BOSULIF tablets.
- •
- Swallow BOSULIF capsules whole. If you cannot swallow BOSULIF capsules whole, tell your healthcare provider.
- •
- If you cannot swallow BOSULIF capsules whole, see the “Instructions for Use” for detailed instructions on how to prepare and give a dose of BOSULIF capsules by opening the capsules and mixing the capsule contents with applesauce or yogurt.
- •
- If you take an antacid or H2 blocker medicine, take it at least 2 hours before or 2 hours after BOSULIF. If you take a Proton Pump Inhibitor (PPI) medicine, talk to your doctor or pharmacist.
- •
- You should avoid grapefruit, grapefruit juice, and supplements that contain grapefruit extract during treatment with BOSULIF. Grapefruit products increase the amount of BOSULIF in your body.
- •
- If you miss a dose of BOSULIF, take it as soon as you remember. If you miss a dose by more than 12 hours, skip that dose and take your next dose at your regular time. Do not take 2 doses at the same time.
- •
- If you take too much BOSULIF, call your doctor or go to the nearest hospital emergency room right away.
What are the possible side effects of BOSULIF?
What are the possible side effects of BOSULIF?
BOSULIF may cause serious side effects, including:- •
- Stomach problems. BOSULIF may cause stomach (abdomen) pain, nausea, diarrhea, vomiting, or blood in your stools. Get medical help right away for any stomach problems.
- •
- Low blood cell counts. BOSULIF may cause low platelet counts (thrombocytopenia), low red blood cell counts (anemia) and low white blood cell counts (neutropenia). Your doctor should do blood tests to check your blood cell counts regularly during your treatment with BOSULIF. Call your doctor right away if you have unexpected bleeding or bruising, blood in your urine or stools, fever, or any signs of an infection.
- •
- Liver problems. Your doctor should do blood tests to check your liver function regularly during your treatment with BOSULIF. Call your doctor right away if your skin or the white part of your eyes turns yellow (jaundice) or you have dark "tea color" urine.
- •
- Heart problems. BOSULIF may cause heart problems, including heart failure and decreased blood flow to the heart which can lead to heart attack. Get medical help right away if you get shortness of breath, weight gain, chest pain, or swelling in your hands, ankles or feet.
- •
- Your body may hold too much fluid (fluid retention). Fluid may build up in the lining of your lungs, the sac around your heart, or your stomach cavity. Get medical help right away if you get any of the following symptoms during your treatment with BOSULIF:
- o
- shortness of breath and cough
- o
- chest pain
- o
- swelling in your hands, ankles, or feet
- o
- swelling all over your body
- o
- weight gain
- •
- Kidney problems. Your doctor should do tests to check your kidney function when you start treatment with BOSULIF and during your treatment. Call your doctor right away if you get any of the following symptoms during your treatment with BOSULIF:
- o
- you urinate more often than normal
- o
- you urinate less often than normal
- o
- you make a much larger amount of urine than normal
- o
- you make a much smaller amount of urine than normal
The most common side effects of BOSULIF in adults and children with CML include:
- •
- diarrhea
- •
- stomach (abdominal) pain
- •
- vomiting
- •
- nausea
- •
- rash
- •
- tiredness
- •
- liver problems
- •
- headache
- •
- fever
- •
- decreased appetite
- •
- respiratory tract infections (infections in nose, throat or lungs)
- •
- constipation
- •
- changes in certain blood tests. Your doctor may do blood tests during treatment with BOSULIF to check for changes
Tell your doctor or get medical help right away if you get respiratory tract infections, loss of appetite, headache, dizziness, back pain, joint pain, rash or itching while taking BOSULIF. These may be symptoms of a severe allergic reaction.
Your doctor may change your dose, temporarily stop, or permanently stop treatment with BOSULIF if you have certain side effects.
BOSULIF may cause fertility problems in females and males. This may affect your ability to have a child. Talk to your doctor if this is a concern for you.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of BOSULIF.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store BOSULIF?
How should I store BOSULIF?
- •
- Store BOSULIF tablets and capsules at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- The BOSLUIF tablets and capsules bottle has a child-resistant closure.
- •
- The BOSULIF tablets bottle contains a desiccant to help keep your medicine dry (protect it from moisture). Keep the desiccant in the bottle. Do not eat the desiccant.
- •
- Store the BOSULIF capsules in the original bottle.
- •
- Ask your doctor or pharmacist about the right way to throw away outdated or unused BOSULIF.
Keep BOSULIF and all medicines out of the reach of children.
General information about the safe and effective use of BOSULIF.
General information about the safe and effective use of BOSULIF.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use BOSULIF for a condition for which it is not prescribed. Do not give BOSULIF to other people even if they have the same symptoms you have. It may harm them. You can ask your doctor or pharmacist for information about BOSULIF that is written for health professionals.
What are the ingredients in BOSULIF?
What are the ingredients in BOSULIF?
Active ingredient:Inactive ingredients:Tablets:Capsules:
LAB-0639-12.0
For more information, go to www.Bosulif.com or www.pfizermedicalinformation.com or call 1-800-438-1985.
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised 9 2023
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
BOSULIF (BAH-su-lif)
(bosutinib)
capsules
This Instructions for Use contains information on how to prepare and give a dose of BOSULIF capsules by opening the capsules and mixing the contents with applesauce or yogurt for people who cannot swallow capsules whole. Read this Instructions for Use before you prepare or give the first dose of BOSULIF, and each time you get a refill. Ask your healthcare provider or pharmacist if you have any questions.
Important information you need to know before preparing a dose of BOSULIF capsules:
- •
- BOSULIF capsules can be opened and the capsules contents mixed with room temperature applesauce or yogurt.
- •
- Only use applesauce or yogurt. Do not mix BOSULIF with other foods.
- •
- Swallow all of the mixture right away, without chewing. Do not store the mixture for later use.
- •
- If you do not swallow the entire BOSULIF mixture, do not mix another dose. Wait until the next day to take your regularly scheduled dose.
- •
- Take the BOSULIF mixture with a full meal.
Preparing a dose of BOSULIF capsules:
Gather the following supplies:
- •
- BOSULIF capsules
- •
- small, clean container
- •
- yogurt or applesauce
- •
- teaspoon for mixing
- •
- disposable gloves
Giving a dose of BOSULIF capsules:
Step 1: Choose a clean, flat work surface. Place all supplies on the work surface.
Step 2: Wash and dry your hands well.
Step 3: Put on disposable gloves
Step 4: Get the prescribed number of BOSULIF capsule(s) needed to prepare the dose.
Step 5: Add the amount of applesauce or yogurt needed for the prescribed dose to the container.
Dose | Amount of Applesauce or Yogurt |
100 mg | 10 mL (2 teaspoons) |
150 mg | 15 mL (3 teaspoons) |
200 mg | 20 mL (4 teaspoons) |
250 mg | 25 mL (5 teaspoons) |
300 mg | 30 mL (6 teaspoons) |
350 mg | 30 mL (6 teaspoons) |
400 mg | 35 mL (7 teaspoons) |
450 mg | 40 mL (8 teaspoons) |
500 mg | 45 mL (9 teaspoons) |
550 mg | 45 mL (9 teaspoons) |
600 mg | 50 mL (10 teaspoons) |
Step 6: Carefully open each of the BOSULIF capsule(s) needed for the dose and empty the entire contents into the applesauce or yogurt. Mix the entire capsule contents with the applesauce or yogurt in the container.
Step 7: Swallow all of the mixture right away, without chewing.
Step 8: Dispose of (throw away) the empty BOSULIF capsule shell(s) in the household trash.
Step 9: Wash teaspoon and the container with soap and warm water.
Step 10: Remove disposable gloves and throw them away in the household trash.
Step 11: Wash and dry your hands.
How should I store BOSULIF capsules?
How should I store BOSULIF capsules?
- •
- Store BOSULIF at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Store the BOSULIF capsules in the original bottle.
- •
- Ask your doctor or pharmacist about the right way to throw away outdated or unused BOSULIF.
Keep BOSULIF and all medicines out of the reach of children.
LAB-0639-12.0
For more information, go to www.Bosulif.com or www.pfizermedicalinformation.com or call 1-800-438-1985.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 9 2023
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Dosage and Administration
Instruct patients to take BOSULIF exactly as prescribed, not to change their dose or to stop taking BOSULIF unless they are told to do so by their doctor. If patients miss a dose beyond 12 hours, they should be advised to take the next scheduled dose at its regular time. A double dose should not be taken to make up for any missed dose. Advise patients to take BOSULIF with food. Patients should be advised: “Swallow tablets whole. Do not crush, break, or cut tablet. Do not touch or handle crushed or broken tablets.” Patients should be advised: “Capsules may be swallowed whole. For those that cannot swallow the capsule whole, the capsule can be opened and the contents mixed with applesauce or yogurt.”
- •
- Gastrointestinal Toxicity
Advise patients that they may experience diarrhea, nausea, vomiting, abdominal pain, or blood in their stools with BOSULIF and to seek medical attention promptly for these symptoms [see Warnings and Precautions (5.1)].
- •
- Myelosuppression
Advise patients of the possibility of developing low blood cell counts and to immediately report fever, any suggestion of infection, or signs or symptoms suggestive of bleeding or easy bruising [see Warnings and Precautions (5.2)].
- •
- Hepatic Toxicity
Advise patients of the possibility of developing liver function abnormalities and to immediately report jaundice [see Warnings and Precautions (5.3)].
- •
- Cardiovascular Toxicity
Advise patients that cardiac failure, left ventricular dysfunction, and cardiac ischemic events have been reported. Advise patients to seek immediate medical attention if any symptoms suggestive of cardiac failure and cardiac ischemia occur, such as shortness of breath, weight gain, or fluid retention [see Warnings and Precautions (5.4)].
- •
- Fluid Retention
Advise patients of the possibility of developing fluid retention (swelling, weight gain, or shortness of breath) and to seek medical attention promptly if these symptoms arise [see Warnings and Precautions (5.5)].
- •
- Renal Toxicity
Advise patients of the possibility of developing renal problems and to immediately report frequent urination, polyuria or oliguria [see Warnings and Precautions (5.6)].
- •
- Adverse Reactions
Advise patients that they may experience other adverse reactions such as respiratory tract infections, rash, fatigue, loss of appetite, headache, dizziness, back pain, arthralgia, pruritus or constipation with BOSULIF and to seek medical attention if symptoms are significant. There is a possibility of anaphylactic shock [see Contraindications (4) and Adverse Reactions (6)].
- •
- Embryo-Fetal Toxicity
Advise females to inform their healthcare provider if they are pregnant or become pregnant. Advise female patients of the risk to a fetus and potential loss of the pregnancy [see Use in Specific Populations (8.1)].
Advise females of reproductive potential, to use effective contraception during treatment and for 2 weeks after receiving the last dose of BOSULIF [see Warnings and Precautions (5.7) and Use in Specific Populations (8.1, 8.3)].
Advise lactating women not to breastfeed during treatment with BOSULIF and for 2 weeks after the last dose [see Use in Specific Populations (8.2)].
- •
- Drug Interactions
Advise patients that BOSULIF and certain other medicines, including over the counter medications or herbal supplements (such as St. John's wort) can interact with each other and may alter the effects of BOSULIF [see Drug Interactions (7)].
PATIENT INFORMATION | ||
BOSULIF (BAH-su-lif) (bosutinib) tablets | BOSULIF (BAH-su-lif) (bosutinib) capsules | |
What is BOSULIF?
It is not known if BOSULIF is safe and effective in children less than 1 year of age with CP Ph+ CML who are newly‑diagnosed or who no longer benefit from or did not tolerate other treatment or in children with AP Ph+ CML or BP Ph+ CML. | ||
Do not take BOSULIF if you are allergic to bosutinib or any of the ingredients in BOSULIF. See the end of this leaflet for a complete list of ingredients of BOSULIF. | ||
Before taking BOSULIF, tell your doctor about all of your medical conditions, including if you:
Tell your doctor about all the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements. When taken together, BOSULIF and certain other medicines can affect each other. | ||
How should I take BOSULIF?
| ||
What are the possible side effects of BOSULIF?
| ||
|
| |
The most common side effects of BOSULIF in adults and children with CML include: | ||
|
| |
Tell your doctor or get medical help right away if you get respiratory tract infections, loss of appetite, headache, dizziness, back pain, joint pain, rash or itching while taking BOSULIF. These may be symptoms of a severe allergic reaction. | ||
How should I store BOSULIF?
Keep BOSULIF and all medicines out of the reach of children. | ||
General information about the safe and effective use of BOSULIF. | ||
What are the ingredients in BOSULIF? 
| ||
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised 9 2023
INSTRUCTIONS FOR USE
BOSULIF (BAH-su-lif)
(bosutinib)
capsules
This Instructions for Use contains information on how to prepare and give a dose of BOSULIF capsules by opening the capsules and mixing the contents with applesauce or yogurt for people who cannot swallow capsules whole. Read this Instructions for Use before you prepare or give the first dose of BOSULIF, and each time you get a refill. Ask your healthcare provider or pharmacist if you have any questions.
Important information you need to know before preparing a dose of BOSULIF capsules:
- •
- BOSULIF capsules can be opened and the capsules contents mixed with room temperature applesauce or yogurt.
- •
- Only use applesauce or yogurt. Do not mix BOSULIF with other foods.
- •
- Swallow all of the mixture right away, without chewing. Do not store the mixture for later use.
- •
- If you do not swallow the entire BOSULIF mixture, do not mix another dose. Wait until the next day to take your regularly scheduled dose.
- •
- Take the BOSULIF mixture with a full meal.
Preparing a dose of BOSULIF capsules:
Gather the following supplies:
- •
- BOSULIF capsules
- •
- small, clean container
- •
- yogurt or applesauce
- •
- teaspoon for mixing
- •
- disposable gloves
Giving a dose of BOSULIF capsules:
Step 1: Choose a clean, flat work surface. Place all supplies on the work surface.
Step 2: Wash and dry your hands well.
Step 3: Put on disposable gloves
Step 4: Get the prescribed number of BOSULIF capsule(s) needed to prepare the dose.
Step 5: Add the amount of applesauce or yogurt needed for the prescribed dose to the container.
Dose | Amount of Applesauce or Yogurt |
100 mg | 10 mL (2 teaspoons) |
150 mg | 15 mL (3 teaspoons) |
200 mg | 20 mL (4 teaspoons) |
250 mg | 25 mL (5 teaspoons) |
300 mg | 30 mL (6 teaspoons) |
350 mg | 30 mL (6 teaspoons) |
400 mg | 35 mL (7 teaspoons) |
450 mg | 40 mL (8 teaspoons) |
500 mg | 45 mL (9 teaspoons) |
550 mg | 45 mL (9 teaspoons) |
600 mg | 50 mL (10 teaspoons) |
Step 6: Carefully open each of the BOSULIF capsule(s) needed for the dose and empty the entire contents into the applesauce or yogurt. Mix the entire capsule contents with the applesauce or yogurt in the container.
Step 7: Swallow all of the mixture right away, without chewing.
Step 8: Dispose of (throw away) the empty BOSULIF capsule shell(s) in the household trash.
Step 9: Wash teaspoon and the container with soap and warm water.
Step 10: Remove disposable gloves and throw them away in the household trash.
Step 11: Wash and dry your hands.
How should I store BOSULIF capsules?
- •
- Store BOSULIF at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Store the BOSULIF capsules in the original bottle.
- •
- Ask your doctor or pharmacist about the right way to throw away outdated or unused BOSULIF.
Keep BOSULIF and all medicines out of the reach of children.
LAB-0639-12.0
For more information, go to www.Bosulif.com or www.pfizermedicalinformation.com or call 1-800-438-1985.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 9 2023
Highlights
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BOSULIF safely and effectively. See full prescribing information for BOSULIF. BOSULIF (bosutinib) tablets, for oral use BOSULIF (bosutinib) capsules, for oral use Initial U.S. Approval: 2012 RECENT MAJOR CHANGES
INDICATIONS AND USAGEBOSULIF is a kinase inhibitor indicated for the treatment of DOSAGE AND ADMINISTRATION
CONTRAINDICATIONSHypersensitivity to BOSULIF. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 9/2023 |
Indications and Usage
1 INDICATIONS AND USAGE
BOSULIF is indicated for the treatment of:
- •
- Adult and pediatric patients 1 year of age and older with chronic phase (CP) Philadelphia chromosome-positive chronic myelogenous leukemia (Ph+ CML), newly-diagnosed or resistant or intolerant to prior therapy [see Clinical Studies (14.1, 14.2, 14.3)].
- •
- Adult patients with accelerated phase (AP), or blast phase (BP) Ph+ CML with resistance or intolerance to prior therapy [see Clinical Studies (14.2)].
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage is taken orally once daily with food. Swallow tablets whole. Do not cut, crush, break or chew tablets. Continue treatment with BOSULIF until disease progression or intolerance to therapy.
Capsules may be swallowed whole. For patients who are unable to swallow a whole capsule(s), each capsule can be opened and the contents mixed with applesauce or yogurt. Mixing the capsule contents with applesauce or yogurt cannot be considered a substitute of a proper meal.
If a dose is missed beyond 12 hours, the patient should skip the dose and take the usual prescribed dose on the following day.
Dosage in Adult Patients with Newly-Diagnosed CP Ph+ CML
The recommended dosage of BOSULIF is 400 mg orally once daily with food.
Dosage in Adult Patients with CP, AP, or BP Ph+ CML with Resistance or Intolerance to Prior Therapy
The recommended dosage of BOSULIF is 500 mg orally once daily with food.
Dosage in Pediatric Patients with Newly-Diagnosed CP Ph+ CML or with CP Ph+ CML with Resistance or Intolerance to Prior Therapy
The recommended dose of BOSULIF for pediatric patients with newly-diagnosed CP Ph+ CML is 300 mg/m2 orally once daily with food and the recommended dosage for pediatric patients with CP Ph+ CML that is resistant or intolerant to prior therapy is 400 mg/m2 orally once daily with food and dose recommendations are provided in Table 1. As appropriate, the desired dose can be attained by combining different strengths of BOSULIF tablets or capsules.
BSA* | Newly-Diagnosed Recommended Dose (Once Daily) | Resistant or Intolerant Recommended Dose (Once Daily) |
< 0.55 m2 | 150 mg | 200 mg |
0.55 to < 0.63 m2 | 200 mg | 250 mg |
0.63 to < 0.75 m2 | 200 mg | 300 mg |
0.75 to < 0.9 m2 | 250 mg | 350 mg |
0.9 to < 1.1 m2 | 300 mg | 400 mg |
≥ 1.1 m2 | 400 mg† | 500 mg† |
Preparation Instructions for BOSULIF Capsules Mixed with Applesauce or Yogurt
For patients who are unable to swallow capsules, the contents of the capsules can be mixed with applesauce or yogurt. Remove the required number of capsules from the container to prepare the dose as instructed and the amount of either room temperature applesauce or yogurt in a clean container. Carefully open each capsule, add the entire capsule content of each capsule into the applesauce or yogurt, then mix the entire dose into the applesauce or yogurt. Patients should immediately consume the full mixture in its entirety, without chewing. Do not store the mixture for later use. If the entire preparation is not swallowed do not take an additional dose. Wait until the next day to resume dosing.
Dose | Volume of Applesauce or Yogurt |
100 mg | 10 mL (2 teaspoons) |
150 mg | 15 mL (3 teaspoons) |
200 mg | 20 mL (4 teaspoons) |
250 mg | 25 mL (5 teaspoons) |
300 mg | 30 mL (6 teaspoons) |
350 mg | 30 mL (6 teaspoons) |
400 mg | 35 mL (7 teaspoons) |
450 mg | 40 mL (8 teaspoons) |
500 mg | 45 mL (9 teaspoons) |
550 mg | 45 mL (9 teaspoons) |
600 mg | 50 mL (10 teaspoons) |
2.2 Dose Escalation
In clinical studies of adult patients with Ph+ CML, dose escalation by increments of 100 mg once daily to a maximum of 600 mg once daily was allowed in patients who did not achieve or maintain a hematologic, cytogenetic, or molecular response and who did not have Grade 3 or higher adverse reactions at the recommended starting dosage.
In pediatric patients with BSA <1.1 m2 and an insufficient response after 3 months consider increasing dose by 50 mg increments up to maximum of 100 mg above starting dose. Dose increases for insufficient response in pediatric patients with BSA ≥1.1 m2 can be conducted similarly to adult recommendations in 100 mg increments.
The maximum dose in pediatric and adult patients is 600 mg once daily.
2.3 Dosage Adjustments for Non-Hematologic Adverse Reactions
Elevated liver transaminases: If elevations in liver transaminases greater than 5×institutional upper limit of normal (ULN) occur, withhold BOSULIF until recovery to less than or equal to 2.5×ULN and resume at 400 mg once daily thereafter. If recovery takes longer than 4 weeks, discontinue BOSULIF. If transaminase elevations greater than or equal to 3×ULN occur concurrently with bilirubin elevations greater than 2×ULN and alkaline phosphatase less than 2×ULN (Hy's law case definition), discontinue BOSULIF [see Warnings and Precautions (5.3)].
Diarrhea: For National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) Grade 3–4 diarrhea (increase of greater than or equal to 7 stools/day over baseline/pretreatment), withhold BOSULIF until recovery to Grade less than or equal to 1. BOSULIF may be resumed at 400 mg once daily [see Warnings and Precautions (5.1)].
For other clinically significant, moderate or severe non-hematological toxicity, withhold BOSULIF until the toxicity has resolved, then consider resuming BOSULIF at a dose reduced by 100 mg taken once daily. If clinically appropriate, consider re-escalating the dose of BOSULIF to the starting dose taken once daily.
In pediatric patients, dose adjustments for non-hematologic toxicities can be conducted similarly to adults, however the dose reduction increments may differ. For pediatric patients with BSA <1.1 m2, reduce dose by 50 mg initially followed by additional 50 mg increment if the adverse reaction (AR) persists. For pediatric patients with BSA ≥1.1 m2 or greater, reduce dose similarly to adults.
2.4 Dosage Adjustments for Myelosuppression
Dose reductions for severe or persistent neutropenia and thrombocytopenia are described below (Table 3).
| |
ANC* less than 1000×106/L or Platelets less than 50,000×106/L | Withhold BOSULIF until ANC greater than or equal to1000×106/L and platelets greater than or equal to 50,000×106/L.
|
2.5 Dosage Adjustments for Renal Impairment or Hepatic Impairment
The recommended starting doses for patients with renal and hepatic impairment are described in Table 4 below.
| Recommended Starting Dosage | |||
|---|---|---|---|
| [see Use in Specific Populations (8.6, 8.7) and Clinical Pharmacology (12.3)]. | |||
| Newly-diagnosed chronic phase Ph+ CML | Chronic, accelerated, or blast phase Ph+ CML with resistance or intolerance to prior therapy | |
Normal renal and hepatic function | 400 mg daily | 500 mg daily | |
Renal impairment | |||
| Creatinine clearance 30 to 50 mL/min | 300 mg daily | 400 mg daily |
| Creatinine clearance less than 30 mL/min | 200 mg daily | 300 mg daily |
Hepatic impairment | |||
| Mild (Child-Pugh A), Moderate (Child-Pugh B) or Severe (Child-Pugh C) | 200 mg daily | 200 mg daily |
| [see Use in Specific Populations (8.6, 8.7) and Clinical Pharmacology (12.3)]. | ||||
| ||||
| Newly-Diagnosed CP Ph+ CML Recommended Starting Dose (Once Daily) By Organ Function | |||
Pediatric Patients by Separated BSA* Band | Normal renal and hepatic function | Renal Impairment: Creatinine clearance 30 to 50 mL/min | Renal Impairment: Creatinine clearance less than 30 mL/min | Hepatic Impairment: Mild (Child-Pugh A), Moderate (Child-Pugh B) or Severe (Child-Pugh C) |
Pediatric < 0.55 m2 | 150 mg | 100 mg | 100 mg | 100 mg |
Pediatric 0.55 to < 0.63 m2 | 200 mg | 150 mg | 100 mg | 100 mg |
Pediatric 0.63 to < 0.75 m2 | 200 mg | 150 mg | 100 mg | 100 mg |
Pediatric 0.75 to < 0.9 m2 | 250 mg | 200 mg | 150 mg | 100 mg |
Pediatric 0.9 to < 1.1 m2 | 300 mg | 200 mg | 200 mg | 150 mg |
Pediatric ≥ 1.1 m2 | 400 mg | 300 mg | 200 mg | 200 mg |
| CP Ph+ CML with Resistance or Intolerance to Prior Therapy Recommended Starting Dose (Once Daily) By Organ Function | |||
Pediatric Patients by Separated BSA* Band | Normal renal and hepatic function | Renal Impairment: Creatinine clearance 30 to 50 mL/min | Renal Impairment: Creatinine clearance less than 30 mL/min | Hepatic Impairment: Mild (Child-Pugh A), Moderate (Child-Pugh B) or Severe (Child-Pugh C) |
Pediatric < 0.55 m2 | 200 mg | 150 mg | 100 mg | 100 mg |
Pediatric 0.55 to < 0.63 m2 | 250 mg | 200 mg | 150 mg | 100 mg |
Pediatric 0.63 to < 0.75 m2 | 300 mg | 200 mg | 200 mg | 150 mg |
Pediatric 0.75 to < 0.9 m2 | 350 mg | 250 mg | 200 mg | 150 mg |
Pediatric 0.9 to < 1.1 m2 | 400 mg | 300 mg | 250 mg | 200 mg |
Pediatric ≥ 1.1 m2 | 500 mg | 400 mg | 300 mg | 200 mg |
Dosage Forms and Strengths
3 DOSAGE FORMS AND STRENGTHS
Tablets:
- •
- 100 mg: yellow, oval, biconvex, film-coated tablets debossed with "Pfizer" on one side and "100" on the other.
- •
- 400 mg: orange, oval, biconvex, film-coated tablets debossed with "Pfizer" on one side and "400" on the other.
- •
- 500 mg: red, oval, biconvex, film-coated tablets debossed with "Pfizer" on one side and "500" on the other.
Capsules:
- •
- 50 mg: white body/orange cap with “BOS 50” printed on the body and “Pfizer” printed on the cap in black ink.
- •
- 100 mg: white body/brownish-red cap with “BOS 100” printed on the body and “Pfizer” printed on the cap in black ink.
Contraindications
4 CONTRAINDICATIONS
BOSULIF is contraindicated in patients with a history of hypersensitivity to BOSULIF. Reactions have included anaphylaxis [see Adverse Reactions (6.1)].
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Gastrointestinal Toxicity
Diarrhea, nausea, vomiting, and abdominal pain occur with BOSULIF treatment. Monitor and manage patients using standards of care, including antidiarrheals, antiemetics, and fluid replacement.
In the randomized clinical trial in adult patients with newly-diagnosed Ph+ CML, the median time to onset for diarrhea (all grades) was 4 days and the median duration per event was 3 days.
Among 546 adult patients in a single-arm study in patients with CML who were resistant or intolerant to prior therapy, the median time to onset for diarrhea (all grades) was 2 days and the median duration per event was 2 days. Among the patients who experienced diarrhea, the median number of episodes of diarrhea per patient during treatment with BOSULIF was 3 (range 1–268).
Among 49 pediatric patients with newly-diagnosed CP Ph+ CML or who had CP Ph+ CML that was resistant or intolerant to prior therapy, the median time to onset for diarrhea (all grades) was 2 days and the duration was 2 days. Among patients who experienced diarrhea, the median number of episodes of diarrhea per patient during treatment with BOSULIF was 2 (range 1 – 198).
To manage gastrointestinal toxicity, withhold, dose reduce, or discontinue BOSULIF as necessary [see Dosage and Administration (2.3) and Adverse Reactions (6)].
5.2 Myelosuppression
Thrombocytopenia, anemia and neutropenia occur with BOSULIF treatment. Perform complete blood counts weekly for the first month of therapy and then monthly thereafter, or as clinically indicated. To manage myelosuppression, withhold, dose reduce, or discontinue BOSULIF as necessary [see Dosage and Administration (2.4) and Adverse Reactions (6)].
5.3 Hepatic Toxicity
Bosutinib may cause elevations in serum transaminases (alanine aminotransferase [ALT], aspartate aminotransferase [AST]).
Two cases consistent with drug induced liver injury (defined as concurrent elevations in ALT or AST greater than or equal to 3×ULN with total bilirubin greater than 2×ULN and alkaline phosphatase less than 2×ULN) have occurred without alternative causes. This represented 2 out 1711 patients in BOSULIF clinical trials.
In the 268 adult patients from the safety population in the randomized clinical trial in patients with newly-diagnosed CML in the BOSULIF treatment group, the incidence of ALT elevation was 68.3% and increased AST was 56%. Of patients who experienced increased transaminases of any grade, 73% experienced their first increase within the first 3 months. The median time to onset of increased ALT and AST was 29 and 56 days, respectively, and the median duration was 19 and 15 days, respectively.
Among the 546 adult patients in a single-arm study in patients with CML who were resistant or intolerant to prior therapy, the incidence of increased ALT was 53.3% and AST elevation was 46.7%. Sixty percent of the patients experienced an increase in either ALT or AST. Most cases of transaminase elevations in this study occurred early in treatment; of patients who experienced increased transaminases of any grade, more than 81% experienced their first increase within the first 3 months. The median time to onset of increased ALT and AST was 22 and 29 days, respectively, and the median duration for each was 21 days.
Among 49 pediatric patients with newly‑diagnosed CP Ph+ CML or who had CP Ph+ CML that was resistant or intolerant to prior therapy, the incidence based on laboratory data that worsened from baseline of increased ALT was 59% and of increased AST 51%. Seventy-six percent of the patients experienced an increase in either ALT or AST. Most cases of increased transaminases occurred early in treatment; of patients who experienced increased transaminases of any grade, 84% of patients experienced their first increases within the first 3 months. The median time to onset for adverse reactions of increased ALT and AST was 22 and 15 days, respectively. The median duration for adverse reactions of Grade 3 or 4 increased ALT or AST was 26 and 12 days, respectively.
Perform hepatic enzyme tests monthly for the first 3 months of BOSULIF treatment and as clinically indicated. In patients with transaminase elevations, monitor liver enzymes more frequently. Withhold, dose reduce, or discontinue BOSULIF as necessary [see Dosage and Administration (2.3) and Adverse Reactions (6)].
5.4 Cardiovascular Toxicity
BOSULIF can cause cardiovascular toxicity including cardiac failure, left ventricular dysfunction, and cardiac ischemic events. Cardiac failure events occurred more frequently in previously treated patients than in patients with newly diagnosed CML and were more frequent in patients with advanced age or risk factors, including previous medical history of cardiac failure. Cardiac ischemic events occurred in both previously treated patients and in patients with newly diagnosed CML and were more common in patients with coronary artery disease risk factors, including history of diabetes, body mass index greater than 30, hypertension, and vascular disorders.
In a randomized study of adult patients with newly diagnosed CML, cardiac failure occurred in 1.9% of patients treated with BOSULIF compared to 0.8% of patients treated with imatinib. Cardiac ischemic events occurred in 4.9% of patients treated with BOSULIF compared to 0.8% of patients treated with imatinib.
In a single-arm study in adult patients with CML who were resistant or intolerant to prior therapy, cardiac failure was observed in 5.3% of patients and cardiac ischemic events were observed in 4.9% of patients treated with BOSULIF.
Among 49 pediatric patients with newly diagnosed CP Ph+ CML or who had CP Ph+ CML that was resistant or intolerant to prior therapy, 4 (8%) patients had Grade 1-2 cardiac events, including tachycardia (n=2), angina pectoris, right bundle branch block, and sinus tachycardia (n=1 each).
Monitor patients for signs and symptoms consistent with cardiac failure and cardiac ischemia and treat as clinically indicated. Interrupt, dose reduce, or discontinue BOSULIF as necessary [see Dosage and Administration (2.3)].
5.5 Fluid Retention
Fluid retention occurs with BOSULIF and may manifest as pericardial effusion, pleural effusion, pulmonary edema, and/or peripheral edema.
In the randomized clinical trial of 268 adult patients with newly-diagnosed CML in the bosutinib treatment group, 3 patients (1.1%) experienced severe fluid retention of Grade 3, 1 patient experienced Grade 3 pericardial effusion, and 2 patients experienced Grade 3 pleural effusion. Among 546 adult patients in a single-arm study in patients with Ph+ CML who were resistant or intolerant to prior therapy, Grade 3 or 4 fluid retention was reported in 30 patients (6%). Some patients experienced more than one fluid retention event. Specifically, 24 patients experienced Grade 3 or 4 pleural effusions, 9 patients experienced Grade 3 or Grade 4 pericardial effusions, and 6 patients experienced Grade 3 edema.
Among 49 pediatric patients with newly diagnosed CP Ph+ CML or who had CP Ph+ CML that was resistant or intolerant to prior therapy, Grade 1-2 pericardial effusion, peripheral edema, and face edema were reported in 1 patient each.
Monitor and manage patients using standards of care. Interrupt, dose reduce or discontinue BOSULIF as necessary [see Dosage and Administration (2.3) and Adverse Reactions (6)].
5.6 Renal Toxicity
An on-treatment decline in estimated glomerular filtration rate (eGFR) has occurred in patients treated with BOSULIF. Table 5 identifies the shift from baseline to lowest observed eGFR during BOSULIF therapy for patients in the pooled leukemia studies regardless of line of therapy. The median duration of therapy with BOSULIF was approximately 24 months (range, 0.03 to 155) for patients in these studies.
| Baseline | Follow-Up | ||||||
|---|---|---|---|---|---|---|---|
| Abbreviations: eGFR=estimated glomerular filtration rate; N/n=number of patients. Notes: eGFR was calculated using Modification in Diet in Renal Disease method (MDRD). Notes: Grading is based on Kidney Disease Improving Global Outcomes (KDIGO) Classification by eGFR: Normal: greater than or equal to 90, Mild: 60 to less than 90, Mild to Moderate: 45 to less than 60, Moderate to Severe: 30 to less than 45, Severe: 15 to less than 30, Kidney Failure: less than 15 ml/min/1.73 m2. | |||||||
| |||||||
Renal Function Status | N | Normal | Mild | Mild to Moderate | Moderate to Severe | Severe | Kidney Failure |
Normal | 527 | 115 (21.8) | 330 (62.6) | 50 (9.5) | 23 (4.4) | 3 (0.6) | 5 (0.9) |
Mild | 672 | 10 (1.5) | 259 (38.5) | 271 (40.3) | 96 (14.3) | 26 (3.9) | 6 (0.9) |
Mild to Moderate | 137 | 0 | 6 (4.4) | 40 (29.2) | 66 (48.2) | 24 (17.5) | 1 (0.7) |
Moderate to Severe | 33 | 0 | 1 (3.0) | 1 (3.0) | 8 (24.2) | 19 (57.6) | 4 (12.1) |
Severe | 1 | 0 | 0 | 0 | 0 | 0 | 1 (100) |
Total | 1370 | 125 (9.1) | 596 (43.5) | 362 (26.4) | 193 (14.1) | 72 (5.2) | 17 (1.2) |
Overall, 45% of the pediatric patients with newly diagnosed CP Ph+ CML or resistant or intolerant CP Ph+ CML who had normal eGFR at baseline shifted to a maximum of mild, and 40% pediatric patients who had mild eGFR at baseline shifted to a maximum of moderate during treatment.
Monitor renal function at baseline and during therapy with BOSULIF, with particular attention to those patients who have preexisting renal impairment or risk factors for renal dysfunction. Consider dose adjustment in patients with baseline and treatment emergent renal impairment [see Dosage and Administration (2.5)].
5.7 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, BOSULIF can cause fetal harm when administered to a pregnant woman. There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies conducted in rats and rabbits, oral administration of bosutinib during organogenesis caused adverse developmental outcomes, including structural abnormalities, embryo-fetal mortality, and alterations to growth at maternal exposures (AUC) as low as 1.2 times the human exposure at the dose of 500 mg/day. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 2 weeks after the last dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
Adverse Reactions
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- Gastrointestinal toxicity [see Warnings and Precautions (5.1)].
- •
- Myelosuppression [see Warnings and Precautions (5.2)].
- •
- Hepatic toxicity [see Warnings and Precautions (5.3)].
- •
- Cardiovascular toxicity [see Warnings and Precautions (5.4)].
- •
- Fluid retention [see Warnings and Precautions (5.5)].
- •
- Renal toxicity [see Warnings and Precautions (5.6)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reactions, in ≥20% of adults with newly diagnosed CP Ph+ CML or CP, AP, or BP Ph+ CML with resistance or intolerance to prior therapy (N=814) were diarrhea (80%), rash (44%), nausea (44%), abdominal pain (43%), vomiting (33%), fatigue (33%), hepatic dysfunction (33%), respiratory tract infection (25%), pyrexia (24%), and headache (21%).
The most common laboratory abnormalities that worsened from baseline in ≥20% of adults were creatinine increased (93%), hemoglobin decreased (90%), lymphocyte count decreased (72%), platelets decreased (69%), ALT increased (58%), calcium decreased (53%), white blood cell count decreased (52%), absolute neutrophils count decreased (50%), AST increased (50%), glucose increased (46%), phosphorus decreased (44%), urate increased (41%), alkaline phosphatase increased (40%), lipase increased (36%), creatine kinase increased (29%), and amylase increased (24%).
The most common adverse reactions, in ≥20% of pediatric patients (N=49) were diarrhea (82%), abdominal pain (73%), vomiting (55%), nausea (49%), rash (49%), fatigue (37%), hepatic dysfunction (37%), headache (35%), pyrexia (31%), decreased appetite (27%), and constipation (20%).
The most common laboratory abnormalities that worsened from baseline in ≥20% of pediatric patients were creatinine increased (92%), alanine aminotransferase increased (59%), white blood cell count decreased (53%), aspartate aminotransferase increased (51%), platelet count decreased (49%), glucose increased (41%), calcium decreased (31%), hemoglobin decreased (31%), neutrophil count decreased (31%), lymphocyte count decreased (29%), serum amylase increased (27%), and CPK increased (25%).
Adverse Reactions in Adult Patients With Newly-Diagnosed CP CML
The clinical trial randomized and treated 533 patients with newly-diagnosed CP CML to receive BOSULIF 400 mg daily or imatinib 400 mg daily as single agents (Newly-Diagnosed CP CML Study) [see Clinical Studies (14.1)]. The safety population (received at least 1 dose of BOSULIF) included:
- •
- two hundred sixty-eight (268) patients with newly-diagnosed CP CML had a median duration of BOSULIF treatment of 55 months (range: 0.3 to 60 months) and a median dose intensity of 394 mg/day.
Serious adverse reactions occurred in 22% of patients with newly-diagnosed CP CML who received bosutinib. Serious adverse reactions reported in >2% of patients included hepatic dysfunction (4.1%), pneumonia (3.4%), coronary artery disease (3.4%), and gastroenteritis (2.2%). Fatal adverse reactions occurred in 3 patients (1.1%) due to coronary artery disease (0.4%), cardiac failure acute (0.4%), and renal failure (0.4%).
Permanent discontinuation of bosutinib due to an adverse reaction occurred in 20% of patients with newly-diagnosed CP CML who received bosutinib. Adverse reactions which resulted in permanent discontinuation in > 2% of patients included hepatic dysfunction (9%).
Dose modifications (dose interruption or reductions) of bosutinib due to an adverse reaction occurred in 68% of patients with newly-diagnosed CP CML. Adverse reactions which required dose interruptions or reductions in >5% of patients included hepatic dysfunction (27%), thrombocytopenia (16%), diarrhea (16%), lipase increased (10%), neutropenia (7%), abdominal pain (6%), rash (5%).
The most common adverse reactions, in >20% of bosutinib-treated patients with newly-diagnosed CML (N=268) were diarrhea (75%), hepatic dysfunction (45%), rash (40%), abdominal pain (39%), nausea (37%), fatigue (33%), respiratory tract infection (27%), headache (22%), and vomiting (21%).
The most common laboratory abnormalities that worsened from baseline in ≥20% of patients were creatinine increased (94%), hemoglobin decreased (89%), lymphocyte count decreased (84%), ALT increased (68%), platelet count decreased (68%), glucose increased (57%), AST increased (56%), calcium decreased (55%), phosphorus decreased (54%), lipase increased (53%), white blood cell count decreased (50%), absolute neutrophil count decreased (42%), alkaline phosphatase increased (41%), creatine kinase increased (36%), and amylase increased (32%).
Table 7 identifies adverse reactions greater than or equal to 10% for All Grades and Grades 3 or 4 (3/4) for the Phase 3 CP CML safety population.
| Bosutinib 400 mg Chronic Phase CML (N=268) | Imatinib 400 mg Chronic Phase CML (N=265) | ||||
|---|---|---|---|---|---|
| |||||
System Organ Class | Preferred Term | All Grades | Grade 3/4 | All Grades | Grade 3/4 |
Gastrointestinal disorders | Diarrhea | 75 | 9 | 40 | 1 |
Abdominal pain† | 39 | 2 | 27 | 1 | |
Nausea | 37 | 0 | 42 | 0 | |
Vomiting | 21 | 1 | 20 | 0 | |
Constipation | 13 | 0 | 6 | 0 | |
Hepatobiliary disorders | Hepatic dysfunction‡ | 45 | 27 | 15 | 4 |
Skin and subcutaneous tissue disorders | Rash§ | 40 | 2 | 30 | 2 |
Pruritus | 11 | <1 | 4 | 0 | |
General disorders and administration-site conditions | Fatigue¶ | 33 | 1 | 30 | <1 |
Pyrexia | 17 | 1 | 11 | 0 | |
Edema# | 15 | 0 | 46 | 2 | |
Infections and infestations | Respiratory tract infectionÞ | 27 | 1 | 25 | <1 |
Nervous system disorders | Headache | 22 | 1 | 15 | 1 |
Musculoskeletal and connective tissue disorders | Arthralgia | 18 | 1 | 18 | <1 |
Back pain | 12 | <1 | 9 | <1 | |
Respiratory, thoracic, and mediastinal disorders | Cough | 11 | 0 | 10 | 0 |
Dyspnea | 11 | 1 | 6 | 1 | |
Metabolism and nutrition disorders | Decreased appetite | 11 | <1 | 6 | 0 |
Vascular disorders | Hypertensionß | 10 | 5 | 11 | 5 |
In the randomized study in patients with newly-diagnosed CP CML, one patient in the group treated with BOSULIF experienced a Grade 3 QTcF prolongation (>500 msec). Patients with uncontrolled or significant cardiovascular disease including QT interval prolongation were excluded by protocol.
Table 8 identifies the clinically relevant or severe Grade 3/4 laboratory test abnormalities for the Phase 3 newly-diagnosed CML safety population.
| Bosutinib N=268 % | Imatinib N=265 % | |||
|---|---|---|---|---|
| All Grade | Grade 3–4 | All Grade | Grade 3–4 | |
| Abbreviations: ALT=alanine aminotransferase; AST=aspartate aminotransferase; CML=chronic myelogenous leukemia; SGPT=serum glutamic-pyruvic transaminase; SGOT=serum glutamic-oxaloacetic transaminase; N/n=number of patients; ULN=upper limit of normal. Graded using CTCAE v 4.03 | ||||
| ||||
Hematology Parameters | ||||
Platelet Count decreased | 68 | 14 | 60 | 6 |
Absolute Neutrophil Count decreased | 42 | 9 | 65 | 20 |
Hemoglobin decreased | 89 | 9 | 90 | 7 |
White Blood Cell Count decreased | 50 | 6 | 70 | 8 |
Lymphocyte Count decreased | 84 | 12 | 82 | 14 |
Biochemistry Parameters | ||||
SGPT/ALT increased | 68 | 26 | 28 | 3 |
SGOT/AST increased | 56 | 13 | 29 | 3.4 |
Lipase increased | 53 | 19 | 35 | 8 |
Phosphorus decreased | 54 | 9 | 69 | 21 |
Amylase increased | 32 | 3.4 | 18 | 2.3 |
Alkaline Phosphatase increased | 41 | 0 | 43 | 0.4 |
Calcium decreased | 55 | 1.5 | 57 | 1.1 |
Glucose increased | 57 | 3 | 65 | 3.4 |
Creatine Kinase increased | 36 | 3 | 65 | 5 |
Creatinine increased | 94 | 1.1 | 98 | 0.8 |
Adverse Reactions in Adult Patients With Imatinib-Resistant or -Intolerant Ph+ CP, AP, and BP CML
The single-arm clinical trial enrolled patients with Ph+ CP, AP, or BP CML and with resistance or intolerance to prior therapy [see Clinical Studies (14.2)]. The safety population (received at least 1 dose of BOSULIF) included 546 CML patients:
- •
- two hundred eighty-four (284) patients with CP CML previously treated with imatinib only who had a median duration of BOSULIF treatment of 26 months (range: 0.2 to 155 months), and a median dose intensity of 437 mg/day.
- •
- one hundred nineteen (119) patients with CP CML previously treated with both imatinib and at least 1 additional tyrosine kinase inhibitor (TKI) who had a median duration of BOSULIF treatment of 9 months (range: 0.2 to 148 months) and a median dose intensity of 427 mg/day.
- •
- one hundred forty-three (143) patients with advanced phase (AdvP) CML including 79 patients with AP CML and 64 patients with BP CML. In the patients with AP CML and BP CML, the median duration of BOSULIF treatment was 10 months (range: 0.1 to 140 months) and 3 months (range: 0.03 to 71 months), respectively. The median dose intensity was 406 mg/day, and 456 mg/day, in the AP CML and BP CML cohorts, respectively.
Serious adverse reactions occurred in 30% of patients in the safety population of the single-arm trial in patients with CML (N=546) who were resistant or intolerant to prior therapy. Serious adverse reactions reported in >2% of patients included pneumonia (7%), pleural effusion (6%), pyrexia (3.7%), coronary artery disease (3.5%), dyspnea (2.6%), rash (2.2%), thrombocytopenia (2%), abdominal pain (2%), and diarrhea (2%).
Fatal adverse reactions occurred in 12 patients (2.2%) due to coronary artery disease (0.9%), pneumonia (0.4%), respiratory failure (0.4%), gastrointestinal hemorrhage (0.2%), acute kidney injury (0.2%), and acute pulmonary edema (0.2%).
Permanent discontinuation of bosutinib due to an adverse reaction occurred in 22% of patients with CML who were resistant or intolerant to prior therapy. Adverse reactions which resulted in permanent discontinuation in >2% of patients included thrombocytopenia (6%), hepatic dysfunction (3.3%), and neutropenia (2%).
Dose modifications (dose interruption or reductions) of bosutinib due to an adverse reaction occurred in 66% of patients with CML who were resistant or intolerant to prior therapy. Adverse reactions which required dose interruptions or reductions in >5% of patients included thrombocytopenia (24%), diarrhea (14%), rash (13%), hepatic dysfunction (10%), neutropenia (9%), pleural effusion (8%), vomiting (7%), anemia (6%), and abdominal pain (6%).
The most common adverse reactions, in ≥20% of patients in the safety population of the single-arm trial in patients with CML (N=546) who were resistant or intolerant to prior therapy were diarrhea (83%), nausea (47%), rash (46%), abdominal pain (45%), vomiting (39%), fatigue (33%), pyrexia (28%), hepatic dysfunction (27%), respiratory tract infection (24%), cough (23%), and headache (21%).
The most common laboratory abnormalities that worsened from baseline in ≥20% were creatinine increased (93%), hemoglobin decreased (91%), lymphocyte decreased (80%), platelets decreased (69%), absolute neutrophil count (54%), ALT increased (53%), calcium decreased (53%), white blood cell count decreased (52%), urate increased (48%), AST increased (47%), phosphorus decreased (39%), alkaline phosphatase increased (39%), lipase increased (28%), magnesium increased (25%), potassium decreased (24%), potassium increased (23%). See Table 10 for Grade 3/4 laboratory abnormalities.
Table 9 identifies adverse reactions greater than or equal to 10% for All Grades and Grades 3 or 4 for the Phase 1/2 CML safety population based on long-term follow-up.
| CP CML (N=403) | AdvP CML (N=143) | ||||
|---|---|---|---|---|---|
| System Organ Class | Preferred Term | All Grades % | Grade 3/4 % | All Grades % | Grade 3/4 % |
| ADR Definition | |||||
| |||||
Gastrointestinal disorders | Diarrhea | 85 | 10 | 76 | 4 |
Abdominal pain† | 49 | 2 | 36 | 7 | |
Nausea | 47 | 1 | 48 | 2 | |
Vomiting | 38 | 3 | 43 | 3 | |
Constipation | 15 | <1 | 17 | 1 | |
Skin and subcutaneous tissue disorders | Rash‡ | 48 | 9 | 42 | 5 |
Pruritus | 12 | 1 | 7 | 0 | |
General disorders and administration-site conditions | Fatigue | 35 | 3 | 27 | 6 |
Pyrexia | 25 | 1 | 37 | 3 | |
Edema§ | 19 | <1 | 17 | 1 | |
Chest pain¶ | 8 | 1 | 12 | 1 | |
Hepatobiliary disorders | Hepatic dysfunction# | 29 | 11 | 21 | 10 |
Infections and infestations | Respiratory tract infectionÞ | 27 | <1 | 17 | 0 |
Influenzaß | 11 | 1 | 3 | 0 | |
Pneumoniaà | 10 | 4 | 18 | 12 | |
Respiratory, thoracic, and mediastinal disorders | Cough | 24 | 0 | 22 | 0 |
Pleural effusion | 14 | 4 | 9 | 4 | |
Dyspnea | 12 | 2 | 20 | 6 | |
Nervous system disorders | Headache | 21 | 1 | 18 | 4 |
Dizziness | 11 | 0 | 14 | 1 | |
Musculoskeletal and connective tissue disorders | Arthralgia | 19 | 1 | 15 | 0 |
Back pain | 14 | 1 | 8 | 1 | |
Metabolism and nutrition disorders | Decreased appetite | 14 | 1 | 14 | 0 |
Vascular disorders | Hypertensionè | 11 | 3 | 8 | 3 |
In the single-arm study in patients with CML who were resistant or intolerant to prior therapy, 2 patients (0.4%) experienced QTcF interval of greater than 500 milliseconds. Patients with uncontrolled or significant cardiovascular disease including QT interval prolongation were excluded by protocol.
Table 10 identifies the clinically relevant or severe Grade 3/4 laboratory test abnormalities for the safety population of the study in patients with CML who were resistant or intolerant to prior therapy based on long-term follow-up.
| CP CML N=403 % | AdvP CML N=143 % | |||
|---|---|---|---|---|
| All grade | Grade 3/4 | All grade | Grade 3/4 | |
| Abbreviations: AdvP=advanced phase; ALT=alanine aminotransferase; AST=aspartate aminotransferase; CML=chronic myelogenous leukemia; CP=chronic phase; N/n=number of patients; SGPT=serum glutamate-pyruvate transaminase; SGOT=serum glutamate-oxaloacetate aminotransferase; ULN=upper limit of normal. | ||||
| ||||
Hematology Parameters | ||||
Platelet Count decreased | 66 | 26 | 80 | 57 |
Absolute Neutrophil Count decreased | 50 | 16 | 66 | 39 |
Hemoglobin decreased | 89 | 13 | 97 | 38 |
Lymphocyte decreased | 79 | 14 | 82 | 21 |
White Blood Cell Count decreased | 51 | 7 | 57 | 27 |
Biochemistry Parameters | ||||
SGPT/ALT increased | 58 | 11 | 39 | 6 |
SGOT/AST increased | 50 | 5 | 37 | 3.5 |
Lipase increased | 32 | 12 | 19 | 6 |
Phosphorus decreased | 41 | 8 | 33 | 7 |
Total Bilirubin increased | 16 | 0.7 | 22 | 2.8 |
Creatinine increased | 95 | 3 | 87 | 1.4 |
Alkaline Phosphatase increased | 39 | 0 | 39 | 1.4 |
Glucose increased | 42 | 2.7 | 39 | 6 |
Sodium increased | 23 | 0.5 | 11 | 0 |
Sodium decreased | 18 | 2.2 | 27 | 6 |
Calcium decreased | 55 | 4.7 | 45 | 3.5 |
Urate increased | 49 | 6 | 43 | 6 |
Magnesium increased | 27 | 7 | 18 | 4.9 |
Potassium decreased | 22 | 1.7 | 29 | 4.9 |
Potassium increased | 25 | 2.7 | 19 | 2.1 |
Pediatric Patients with Newly-Diagnosed CP Ph+ CML or CP Ph+ CML that is Resistant or Intolerant to Prior Therapy
The safety of BOSULIF was evaluated in BCHILD, a single-arm trial for the treatment of pediatric patients aged 1 year and older with newly-diagnosed CP Ph+ CML or in patients with CP Ph+ CML who are resistant or intolerant to prior therapy [see Clinical Studies (14.3)]. Patients received BOSULIF (n = 49) 300 mg/m2 to 400 mg/m2 orally once daily until disease progression or unacceptable toxicity. The median time on treatment with BOSULIF was 12.2 months (range, 0.2 to 60.9 months). Among patients who received BOSULIF, 77.6% were exposed for 6 months or longer and 51% were exposed for one year or longer.
Permanent discontinuation of BOSULIF due to an adverse reaction occurred in 20% of patients. Adverse reactions which resulted in permanent discontinuation in 2 or more patients included ALT increased (6%), AST increased (4%), diarrhea (4%), fatigue (4%) and rash maculo-papular (4%).
The most common adverse reactions, in ≥20% of BOSULIF-treated pediatric patients were diarrhea, abdominal pain, vomiting, nausea, rash, fatigue, hepatic dysfunction, headache, pyrexia, decreased appetite, and constipation.
Table 11 summarizes the adverse reactions in BCHILD.
| Adverse drug reactions are based on all-causality treatment-emergent adverse reactions. | |||
| The commonality stratification is based on 'All Grades' under Bosutinib 400 mg column. | |||
| 'Grade 3/4 columns indicate maximum toxicity. | |||
| |||
System Organ Class | Preferred Term | BOSULIF Total % | |
All Grades | Grade 3/4 | ||
Gastrointestinal disorders | Diarrhea | 82 | 12 |
Abdominal pain* | 73 | 4 | |
Vomiting | 55 | 6 | |
Nausea | 49 | 2 | |
Constipation | 20 | 0 | |
Skin and subcutaneous tissue disorders | Rash† | 49 | 8 |
Hepatobiliary disorders | Hepatic dysfunction‡ | 37 | 14 |
General disorders and administration-site conditions | Fatigue§ | 37 | 4 |
Pyrexia | 31 | 4 | |
Nervous system disorders | Headache | 35 | 2 |
Metabolism and nutrition disorders | Decreased appetite | 27 | 2 |
Infections and infestations | Respiratory tract infection¶ | 12 | 2 |
The most common laboratory abnormalities that worsened from baseline in ≥20% of patients were creatinine increased, alanine aminotransferase increased, white blood cell count decreased, aspartate aminotransferase increased, platelet count decreased, glucose increased, calcium decreased, hemoglobin decreased, neutrophil count decreased, lymphocyte count decreased, serum amylase increased and CPK increased.
Table 12 summarizes laboratory test abnormalities in BCHILD.
| Grades are defined using CTCAE V4.03. Based on CTCAE grading without regard to fasting status for 'Hyperglycemia' lab parameter. | ||
| Includes data up to 28 days after last dose of study treatment. | ||
BOSULIF (N= 49) | ||
All Grade | Grade 3/4 | |
% | % | |
Creatinine increased | 92 | 0 |
Alanine aminotransferase increased | 59 | 14 |
White blood cell count decreased | 53 | 4 |
Aspartate aminotransferase increased | 51 | 6 |
Platelet count decreased | 49 | 18 |
Glucose increased | 41 | 0 |
Calcium decreased | 31 | 0 |
Hemoglobin decreased | 31 | 8 |
Neutrophil count decreased | 31 | 12 |
Lymphocyte count decreased | 29 | 2 |
Serum amylase increased | 27 | 4 |
CPK increased | 25 | 0 |
Additional Adverse Reactions From Multiple Clinical Trials
The following adverse reactions were reported in patients in clinical trials with BOSULIF (less than 10% of BOSULIF-treated patients). They represent an evaluation of the adverse reaction data from all 1372 patients with leukemia who received at least 1 dose of single-agent BOSULIF. These adverse reactions are presented by system organ class and are ranked by frequency. These adverse reactions are included based on clinical relevance and ranked in order of decreasing seriousness within each category.
Blood and Lymphatic System Disorders: 0.1% and less than 1% - Febrile neutropenia
Cardiac Disorders: 1% and less than 10% - Pericardial effusion; 0.1% and less than 1% - Pericarditis
Ear and Labyrinth Disorders: 1% and less than 10% - Tinnitus
Endocrine Disorders: 1% and less than 10% - Hypothyroidism; 0.1% and less than 1% - Hyperthyroidism
Gastrointestinal Disorders: 1% and less than 10% - Gastritis, Pancreatitis (includes Edematous pancreatitis, Pancreatic enzymes increased, Pancreatitis, Pancreatitis acute, Pancreatitis chronic), Gastrointestinal hemorrhage (includes Anal hemorrhage, Gastric hemorrhage, Gastrointestinal hemorrhage, Intestinal hemorrhage, Lower gastrointestinal hemorrhage, Rectal hemorrhage, Upper gastrointestinal hemorrhage)
General Disorders and Administrative Site Conditions: 1% and less than 10% - Pain
Immune System Disorders: 1% and less than 10% - Drug hypersensitivity; 0.1% and less than 1% - Anaphylactic shock
Infections and Infestations: 1% and less than 10% - Bronchitis
Investigations: 1% and less than 10% - Electrocardiogram QT prolonged (includes Electrocardiogram QT prolonged, Long QT syndrome)
Metabolism and Nutrition Disorders: 1% and less than 10% - Dehydration
Musculoskeletal and Connective Tissue Disorders: 1% and less than 10% - Myalgia
Nervous System Disorders: 1% and less than 10% - Dysgeusia
Renal and Urinary Disorders: 1% and less than 10% - Acute kidney injury, Renal impairment, Renal failure
Respiratory, Thoracic and Mediastinal Disorders: 1% and less than 10% - Pulmonary hypertension (includes Pulmonary hypertension, Pulmonary arterial hypertension, Pulmonary arterial pressure increased); 0.1% and less than 1% - Acute pulmonary edema (includes Acute pulmonary edema, Pulmonary edema), Interstitial lung disease, Respiratory failure
Skin and Subcutaneous Disorders: 0.1% and less than 1% - Erythema multiforme
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of BOSULIF. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Thrombotic microangiopathy
Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome
Drug Interactions
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on BOSULIF
Strong or Moderate CYP3A Inhibitors
Avoid the concomitant use of strong or moderate CYP3A inhibitors with BOSULIF. Bosutinib is a CYP3A substrate. Concomitant use with a strong or moderate CYP3A inhibitor increases bosutinib Cmax and AUC [see Clinical Pharmacology (12.3)] which may increase the risk of toxicities.
Strong CYP3A Inducers
Avoid the concomitant use of strong CYP3A inducers with BOSULIF. Bosutinib is a CYP3A substrate. Concomitant use with a strong CYP3A inducer decreases bosutinib Cmax and AUC [see Clinical Pharmacology (12.3)] which may reduce BOSULIF efficacy.
Proton Pump Inhibitors (PPI)
As an alternative to PPIs, use short-acting antacids or H2 blockers and separate dosing by more than 2 hours from BOSULIF dosing. Bosutinib displays pH dependent aqueous solubility, Concomitant use with a PPI decreases bosutinib Cmax and AUC [see Clinical Pharmacology (12.3)] which may reduce BOSULIF efficacy.
Use in Specific Populations
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action, BOSULIF can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)].
There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies conducted in rats and rabbits, oral administration of bosutinib during organogenesis caused adverse developmental outcomes, including structural abnormalities, embryo-fetal mortality, and alterations to growth at maternal exposures (AUC) as low as 1.2 times the human exposure at the dose of 500 mg/day (see Data). Advise pregnant women of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies are 2–4% and 15–20%, respectively.
Animal Data
In a rat fertility and early embryonic development study, bosutinib was administered orally to female rats for approximately 3 to 6 weeks, depending on day of mating (2 weeks prior to cohabitation with untreated breeder males until gestation day [GD] 7). Increased embryonic resorptions occurred at greater than or equal to 10 mg/kg/day of bosutinib (1.6 and 1.2 times the human exposure at the recommended doses of 400 or 500 mg/day, respectively), and decreased implantations and reduced number of viable embryos at 30 mg/kg/day of bosutinib (3.4 and 2.5 times the human exposure at the recommended doses of 400 or 500 mg/day, respectively).
In an embryo-fetal development study conducted in rabbits, bosutinib was administered orally to pregnant animals during the period of organogenesis at doses of 3, 10, and 30 mg/kg/day. At the maternally-toxic dose of 30 mg/kg/day of bosutinib, there were fetal anomalies (fused sternebrae, and 2 fetuses had various visceral observations), and an approximate 6% decrease in fetal body weight. The dose of 30 mg/kg/day resulted in exposures (AUC) approximately 5.1 and 3.8 times the human exposures at the recommended doses of 400 and 500 mg/day, respectively.
Fetal exposure to bosutinib-derived radioactivity during pregnancy was demonstrated in a placental-transfer study in pregnant rats. In a rat pre- and postnatal development study, bosutinib was administered orally to pregnant animals during the period of organogenesis through lactation day 20 at doses of 10, 30, and 70 mg/kg/day. Reduced number of pups born occurred at greater than or equal to 30 mg/kg/day bosutinib (3.4 and 2.5 times the human exposure at the recommended doses of 400 or 500 mg/day, respectively), and increased incidence of total litter loss and decreased growth of offspring after birth occurred at 70 mg/kg/day bosutinib (6.9 and 5.1 times the human exposure at the recommended doses of 400 or 500 mg/day, respectively).
8.2 Lactation
Risk Summary
No data are available regarding the presence of bosutinib or its metabolites in human milk or its effects on a breastfed child or on milk production. However, bosutinib is present in the milk of lactating rats. Because of the potential for serious adverse reactions in a nursing child, breastfeeding is not recommended during treatment with BOSULIF and for 2 weeks after the last dose.
8.3 Females and Males of Reproductive Potential
Based on findings from animal studies, BOSULIF can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy
Females of reproductive potential should have a pregnancy test prior to starting treatment with BOSULIF.
Contraception
Females
Advise females of reproductive potential to use effective contraception (methods that result in less than 1% pregnancy rates) during treatment with BOSULIF and for 2 weeks after the last dose.
Infertility
The risk of infertility in females or males of reproductive potential has not been studied in humans. Based on findings from animal studies, BOSULIF may cause reduced fertility in females and males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of BOSULIF have been established in pediatric patients 1 year of age and older with newly-diagnosed CP Ph+ CML and CP Ph+ CML that is resistant or intolerant to prior therapy.
Use of BOSULIF for these indications is based on data from BCHILD [NCT04258943]. The study included pediatric patients with newly diagnosed CP Ph+ CML in the following age groups: 2 patients 1 year of age to less than 6 years of age, 3 patients 6 years of age to less than 12 years of age, and 10 patients 12 years of age to less than 17 years of age. The study also included pediatric patients with CP Ph+ CML that was resistant or intolerant to prior therapy in the following age groups: 4 patients 1 year of age to less than 6 years of age, 10 patients 6 years of age to less than 12 years of age, and 10 patients 12 years of age to less than 17 years of age [see Adverse Reactions (6.1) and Clinical Studies (14.3)]. BSA-normalized apparent clearance in 27 pediatric patients aged 4 to <17 years (141.3 L/h/m2) was 29% higher than BSA-normalized apparent clearance in adult patients with CP Ph+ CML (109.2 L/h/m2) [see Clinical Pharmacology (12.3)]. The recommended dosage of BOSULIF in pediatric patients is based on body-surface area (BSA) [see Dosage and Administration (2.1)].
The safety and effectiveness of BOSULIF in pediatric patients younger than 1 year of age with newly diagnosed CP Ph+ CML, pediatric patients younger than 1 year of age with CP Ph+ CML that is resistant or intolerant to prior therapy, and pediatric patients with AP Ph+ CML or BP Ph+ CML have not been established.
8.5 Geriatric Use
In the single-arm study in patients with CML who were resistant or intolerant to prior therapy of BOSULIF in patients with Ph+ CML, 20% were age 65 and over, 4% were 75 and over. Of the 268 patients who received bosutinib in the study for newly diagnosed CML, 20% were age 65 and over, 5% were 75 and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Reduce the BOSULIF starting dose in patients with moderate (creatinine clearance [CLcr] 30 to 50 mL/min, estimated by Cockcroft-Gault (C-G)) and severe (CLcr less than 30 mL/min, C-G) renal impairment at baseline. For patients who have declining renal function while on BOSULIF who cannot tolerate the starting dose, follow dose adjustment recommendations for toxicity [see Dosage and Administration (2.3, 2.5) and Clinical Pharmacology (12.3)]. BOSULIF has not been studied in patients undergoing hemodialysis.
8.7 Hepatic Impairment
Reduce the BOSULIF dosage in patients with hepatic impairment (Child-Pugh A, B, or C) [see Dosage and Administration (2.3, 2.5) and Clinical Pharmacology (12.3)].
Overdosage
Description
11 DESCRIPTION
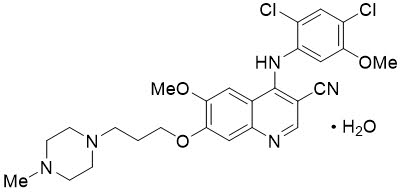
BOSULIF contains bosutinib, a kinase inhibitor. Bosutinib is present as a monohydrate with a chemical name of 3-Quinolinecarbonitrile, 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl) propoxy]-, hydrate (1:1). Its chemical formula is C26H29Cl2N5O3∙H2O (monohydrate); its molecular weight is 548.46 (monohydrate), equivalent to 530.46 (anhydrous). Bosutinib monohydrate has the following chemical structure:
Bosutinib monohydrate is a white to yellowish-tan powder. Bosutinib monohydrate has a pH dependent solubility across the physiological pH range. At or below pH 5, bosutinib monohydrate behaves as a highly soluble compound. Above pH 5, the solubility of bosutinib monohydrate reduces rapidly.
BOSULIF (bosutinib) tablets are supplied for oral administration in 3 strengths: 100 mg, 400 mg and 500 mg. Each strength reflects the equivalent amount of bosutinib content (on anhydrous basis). The tablets contain the following inactive ingredients: croscarmellose sodium, iron oxide red (for 400 mg, and 500 mg tablet) and iron oxide yellow (for 100 mg, and 400 mg tablet), magnesium stearate, microcrystalline cellulose, poloxamer, polyethylene glycol, polyvinyl alcohol, povidone, talc and titanium dioxide.
BOSULIF (bosutinib) capsules are supplied for oral administration in 2 strengths: 50 mg and 100 mg. Each strength reflects the equivalent amount of bosutinib (on anhydrous basis). The capsules contain the following inactive ingredients: croscarmellose sodium, gelatin, magnesium stearate, mannitol, microcrystalline cellulose, poloxamer, povidone, red iron oxide, titanium dioxide, yellow iron oxide. The printing ink contains black iron oxide, potassium hydroxide, propylene glycol, shellac, strong ammonia solution.
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bosutinib is a TKI. Bosutinib inhibits the BCR-ABL kinase that promotes CML; it is also an inhibitor of Src-family kinases including Src, Lyn, and Hck. Bosutinib inhibited 16 of 18 imatinib-resistant forms of BCR-ABL kinase expressed in murine myeloid cell lines. Bosutinib did not inhibit the T315I and V299L mutant cells.
12.2 Pharmacodynamics
A greater likelihood of response and a greater likelihood of safety events were observed with higher bosutinib exposure in clinical studies. The time course of bosutinib pharmacodynamic response has not been fully characterized.
12.3 Pharmacokinetics
Bosutinib pharmacokinetics were assessed following oral dosing with food in adult patients with CML and were presented as geometric mean (CV%), unless otherwise specified.
Bosutinib exhibits dose proportional increases in Cmax and AUC over the oral dose range of 200 to 800 mg (0.33 to 1.3 times the maximum approved recommended dosage of 600 mg). Bosutinib steady state Cmax was 127 ng/mL (31%), Ctrough was 68 ng/mL (39%) and AUC was 2370 ng•h/mL (34%) following multiple oral doses of BOSULIF 400 mg; Bosutinib steady state Cmax was 171 ng/mL (38%), Ctrough was 91 ng/mL (42%) and AUC was 3150 ng•h/mL (38%) following multiple oral doses of BOSULIF 500 mg. No clinically significant differences in the pharmacokinetics of bosutinib were observed following administration of either the tablet or capsule dosage forms of BOSULIF at the same dose, under fed conditions.
Absorption
The median bosutinib (minimum, maximum) time-‑to-Cmax (tmax) was 6.0 (6.0, 6.0) hours following oral administration of a single oral dose of BOSULIF 500 mg with food. The absolute bioavailability was 34% in healthy subjects.
Effect of Food
Bosutinib Cmax increased 1.8-fold and AUC increased 1.7-fold when BOSULIF tablets were given with a high fat meal to healthy subjects compared to administration under fasted condition. Bosutinib Cmax increased 1.6‑fold and AUC increased 1.5-fold when BOSULIF capsules were given with a high fat meal to healthy subjects compared to administration under fasted condition. The high-fat meal (800-1000 total calories) consisted of approximately 150 protein calories, 250 carbohydrate calories, and 500-600 fat calories.
No clinically significant differences in the pharmacokinetics of bosutinib were observed following administration of a BOSULIF capsule that was opened and the contents mixed with applesauce or yogurt immediately before use.
Distribution
The mean (SD) apparent bosutinib volume of distribution is 6080 (1230) L after an oral dose of 500 mg of BOSULIF.
Bosutinib protein binding is 94% in vitro and 96% ex vivo, and is independent of concentration.
Elimination
The mean (SD) bosutinib terminal phase elimination half life (t½) was 22.5 (1.7) hours, and the mean (SD) apparent clearance was 189 (48) L/h following a single oral dose of BOSULIF.
Specific Populations
Patients with Renal Impairment
Bosutinib AUC increased 1.4-fold in subjects with moderate renal impairment (CLcr: 30 to 50 mL/min, estimated by Cockcroft-Gault (C-G)) and increased 1.6-fold in subjects with severe renal impairment (CLcr less than 30 mL/min) following a single oral dose of BOSULIF 200 mg (0.33 times the maximum approved recommended dosage of 600 mg). No clinically significant difference in the pharmacokinetics of bosutinib was observed in subjects with mild renal impairment (CLcr: 51 to 80 mL/min, C-G). BOSULIF has not been studied in patients undergoing hemodialysis.
Patients with Hepatic Impairment
Bosutinib Cmax increased 2.4-fold, 2-fold, and 1.5-fold, and AUC increased 2.3-fold, 2-fold, and 1.9-fold in hepatic impairment Child-Pugh A, B, and C, respectively, following a single oral dose of BOSULIF 200 mg (0.33 times the maximum approved recommended dosage of 600 mg).
Pediatric Patients
The pharmacokinetics of bosutinib in 27 pediatric patients aged 4 to less than 17 years with newly diagnosed CP Ph+ CML or resistant/intolerant CP Ph+ CML were evaluated over the dose range of 300 mg/m2 to 400 mg/m2 administered orally once daily with food. Exposures increased in a dose proportional manner over the dose range of 300 mg/m2 to 400 mg/m2. The bosutinib median (min, max) tmax is approximately 3 hours post-dose (1, 8 hours). In 15 pediatric patients aged 4 to less than 17 years who received 300 mg/m2 daily, steady state Cmax was 159 ng/mL (42%), Ctrough was 49 ng/mL (53%) and AUC was 2027 ng•h/mL (47%). In 6 pediatric patients aged 6 to less than 17 years who received 400 mg/m2 daily, steady state Cmax was 198 ng/mL (37%), Ctrough was 42 ng/mL (105%), and AUC was 2514 ng•h/mL (35%).
An increase in BSA correlated with an increase in apparent clearance and exposure metrics did not significantly differ across BSA or age in pediatric patients following the approved recommended BSA-based dosage.
Drug Interaction Studies
Strong CYP3A Inhibitors:
Bosutinib Cmax increased 5.2-fold and AUC increased 8.6-foldfollowing a single dose of BOSULIF 100 mg (0.17 times the maximum approved recommended dosage) without food when used concomitantly with 400 mg ketoconazole (a strong CYP3A inhibitor) administered over multiple daily doses.
Moderate CYP3A Inhibitors: Bosutinib Cmax increased 1.5-fold and AUC increased 2.0-fold following a single dose of BOSULIF 500 mg with food when administered concomitantly with 125 mg aprepitant (a moderate CYP3A inhibitor).
Strong CYP3A Inducers: Bosutinib Cmax decreased by 86% and AUC decreased by 94% following a single dose of BOSULIF 500 mg with food administered concomitantly with multiple daily doses of 600 mg of rifampin (a strong CYP3A inducer).
Proton Pump Inhibitors: Lansoprazole decreased bosutinib Cmax by 46% and AUC by 26% following a single oral dose of BOSULIF 400 mg without food when used concomitantly with lansoprazole 60 mg (proton pump inhibitor) administered over multiple daily doses. Bosutinib displays pH‑dependent aqueous solubility, in vitro [see Description (11)].
P-gp Substrates: No clinically significant differences in bosutinib pharmacokinetics were observed when used concomitantly with dabigatran etexilate mesylate (a P-glycoprotein (P-gp) substrate).
Nonclinical Toxicology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Bosutinib was not carcinogenic in rats or transgenic mice. The rat 2-year carcinogenicity study was conducted at bosutinib oral doses up to 25 mg/kg in males and 15 mg/kg in females. Exposures at these doses were approximately 1.5 times (males) and 3.1 times (females) the human exposure at the 400 mg dose and 1.2 times (males) and 2.4 times (females) exposure in humans at the 500 mg dose. The 6-month RasH2 transgenic mouse carcinogenicity study was conducted at bosutinib oral doses up to 60 mg/kg.
Bosutinib was not mutagenic or clastogenic in a battery of tests, including the bacteria reverse mutation assay (Ames Test), the in vitro assay using human peripheral blood lymphocytes and the micronucleus test in orally treated male mice.
In a rat fertility study, drug-treated males were mated with untreated females, or untreated males were mated with drug-treated females. Females were administered the drug from pre-mating through early embryonic development. The dose of 70 mg/kg/day of bosutinib resulted in reduced fertility in males as demonstrated by 16% reduction in the number of pregnancies. There were no lesions in the male reproductive organs at this dose. This dose of 70 mg/kg/day resulted in exposure (AUC) in male rats approximately 1.5 times and equal to the human exposure at the recommended doses of 400 and 500 mg/day, respectively. Fertility (number of pregnancies) was not affected when female rats were treated with bosutinib. However, there were increased embryonic resorptions at greater than or equal to 10 mg/kg/day of bosutinib (1.6 and 1.2 times the human exposure at the recommended doses of 400 and 500 mg/day, respectively), and decreased implantations and reduced number of viable embryos at 30 mg/kg/day of bosutinib (3.4 and 2.5 times the human exposure at the recommended doses of 400 or 500 mg/day, respectively).
Clinical Studies
14 CLINICAL STUDIES
14.1 Adult Patients with Newly-Diagnosed CP Ph+ CML
The efficacy of BOSULIF in patients with newly-diagnosed chronic phase Ph+ CML was evaluated in the Bosutinib trial in First-line chrOnic myelogenous leukemia tREatment (BFORE) Trial: "A Multicenter Phase 3, Open-Label Study of Bosutinib Versus Imatinib in Adult Patients With Newly Diagnosed Chronic Phase Chronic Myelogenous Leukemia" [NCT02130557].
The BFORE Trial is a 2-arm, open-label, randomized, multicenter trial conducted to investigate the efficacy and safety of BOSULIF 400 mg once daily alone compared with imatinib 400 mg once daily alone in adult patients with newly-diagnosed CP Ph+ CML. The trial randomized 536 patients (268 in each arm) with Ph+ or Ph- newly-diagnosed CP CML (intent-to-treat [ITT] population) including 487 patients with Ph+ CML harboring b2a2 and/or b3a2 transcripts at baseline and baseline BCR-ABL copies >0 (modified intent-to-treat [mITT] population). Randomization was stratified by Sokal score and geographical region. All patients are being treated and/or followed for up to 5 years (240 weeks). Efficacy was evaluated in the mITT population. The major efficacy outcome measure was major molecular response (MMR) at 12 months (48 weeks) defined as ≤0.1% BCR-ABL ratio on international scale (corresponding to ≥3 log reduction from standardized baseline) with a minimum of 3000 ABL transcripts as assessed by the central laboratory. Additional efficacy outcomes included CCyR by 12 months, defined as the absence of Ph+ metaphases in chromosome banding analysis of ≥20 metaphases derived from bone marrow aspirate or MMR if an adequate cytogenetic assessment was unavailable and MMR by 18 months (72 weeks).
In the mITT population in this study, 57% of patients were males, 78% were Caucasian, and 19% were 65 years or older. The median age was 53 years. At baseline, the distribution of Sokal risk scores was similar in bosutinib and imatinib-treated patients (low risk: 35% and 39%; intermediate risk: 44% and 38%; high risk: 22% and 22%, respectively). After a minimum of 12 months follow-up, 78% of the 246 bosutinib-treated patients and 72% of the 239 imatinib-treated patients were still receiving treatment and with a minimum of 60 months of follow-up, 60% and 60% of patients, respectively, were still receiving treatment. The median treatment duration was 55.1 months for BOSULIF and 55.0 months for imatinib.
The efficacy results from the BFORE trial are summarized in Table 13.
| Response | Bosutinib N=246 n (%) | Imatinib N=241 n (%) | 2-sided p-value |
|---|---|---|---|
| Abbreviations: CCyR=complete cytogenetic response; CI=confidence interval; CMH=Cochran-Mantel-Haenszel; MMR=major molecular response; N/n=number of patients. | |||
| |||
MMR at Month 12 (Week 48) | |||
MMR (%) | 116 (47) | 89 (37) | 0.0200* |
(95% CI) | (41, 53) | (31, 43) | |
CCyR by Month 12 (Week 48) | |||
CCyR (%) | 190 (77) | 160 (66) | |
(95% CI) | (72, 83) | (60, 72) | 0.0075* |
MMR by Month 18 (Week 72) | |||
MMR (%) | 150 (61) | 127 (53) | |
(95% CI) | (55, 67) | (46, 59) | 0.0606* |
The MMR rate at Month 12 for all randomized patients (ITT population) was consistent with the mITT population (47% [95% CI: 41, 53] in the bosutinib treatment group and 36% [95% CI: 30, 42] in the imatinib treatment group; odds ratio of 1.57 [95% CI: 1.10, 2.22]). MMR by Month 60 (Week 240) in the mITT population was 74% (95% CI: 69, 80) in the bosutinib treatment group and 66% (95% CI: 60, 72) in the imatinib treatment group; odds ratio of 1.52 (95% CI: 1.02, 2.25). MMR by Month 60 in the ITT population was also consistent with the mITT population (1.57 [95% CI: 1.08, 2.28]).
After 60 months of follow-up, the median time to MMR in responders was 9.0 months for bosutinib and 11.9 months for imatinib.
By 60 months, the MMR rates in each Sokal risk group for the bosutinib and imatinib-treated patients, respectively, were 78% and 72% for low risk, 74% and 67% for intermediate risk and 68% and 52% for high risk.
After 60 months of follow-up, 6 (2%) bosutinib patients and 7 (3%) imatinib patients transformed to AP CML or BP CML while on treatment.
At 60 months, the estimated overall survival rate was 95% (95% CI: 91, 97) in the bosutinib group and 94% (95% CI: 90, 96) in the imatinib group.
14.2 Adult Patients with Imatinib-Resistant or -Intolerant Ph+ CP, AP, and BP CML
Study 200 (NCT00261846), a single-arm, open-label, multicenter study in patients with CML who were resistant or intolerant to prior therapy was conducted to evaluate the efficacy and safety of BOSULIF 500 mg once daily in patients with imatinib-resistant or -intolerant CML with separate cohorts for CP, AP, and BP disease previously treated with 1 prior TKI (imatinib) or more than 1 TKI (imatinib followed by dasatinib and/or nilotinib). The definition of imatinib resistance included (1) failure to achieve or maintain any hematologic improvement within 4 weeks; (2) failure to achieve a CHR by 3 months, cytogenetic response by 6 months or major cytogenetic response (MCyR) by 12 months; (3) progression of disease after a previous cytogenetic or hematologic response; or (4) presence of a genetic mutation in the BCR-ABL gene associated with imatinib resistance. Imatinib intolerance was defined as inability to tolerate imatinib due to toxicity, or progression on imatinib and inability to receive a higher dose due to toxicity. The definitions of resistance and intolerance to both dasatinib and nilotinib were similar to those for imatinib. The protocol was amended to exclude patients with a known history of the T315I mutation after 396 patients were enrolled in the trial.
The efficacy endpoints for patients with CP CML previously treated with 1 prior TKI (imatinib) were the rate of attaining MCyR by Week 24 and the duration of MCyR. The efficacy endpoints for patients with CP CML previously treated with both imatinib and at least 1 additional TKI were the cumulative rate of attaining MCyR by Week 24 and the duration of MCyR. The efficacy endpoints for patients with previously treated AP and BP CML were confirmed CHR and overall hematologic response (OHR).
The study enrolled 546 patients with CP, AP or BP CML. Of the total patient population 73% were imatinib resistant and 27% were imatinib intolerant. In this trial, 53% of patients were males, 65% were Caucasian, and 20% were 65 years old or older. Of the 546 treated patients, 506 were considered evaluable for cytogenetic or hematologic efficacy assessment. Patients were evaluable for efficacy if they had received at least 1 dose of BOSULIF and had a valid baseline efficacy assessment. Among evaluable patients, there were 262 patients with CP CML previously treated with 1 prior TKI (imatinib), 112 patients with CP CML previously treated with both imatinib and at least 1 additional TKI, and 132 patients with advanced phase CML previously treated with at least 1 TKI.
Median duration of BOSULIF treatment was 26 months in patients with CP CML previously treated with 1 TKI (imatinib), 9 months in patients with CP CML previously treated with imatinib and at least 1 additional TKI, 10 months in patients with AP CML previously treated with at least imatinib, and 3 months in patients with BP CML previously treated with at least imatinib.
The 24 week efficacy and MCyR at any time results are summarized in Table 14.
| Prior Treatment With Imatinib Only (N=262 evaluable) n (%) | Prior Treatment With Imatinib and Dasatinib or Nilotinib (N=112 evaluable) n (%) | |
|---|---|---|
| Abbreviations: CI=confidence interval; CML=chronic myelogenous leukemia; CP=chronic phase; MCyR=major cytogenetic response; N/n=number of patients; Ph+=Philadelphia chromosome positive. | ||
By Week 24 | ||
MCyR | 105 (40.1) | 29 (25.9) |
(95% CI) | (34.1, 46.3) | (18.1, 35.0) |
MCyR any time | 156 (59.5) | 45 (40.2) |
(53.3, 65.5) | (31.0, 49.9) | |
The long-term follow-up data analysis was based on a minimum of 60 months for patients with CP CML treated with 1 prior TKI (imatinib) and a minimum of 48 months for patients with CP CML treated with imatinib and at least 1 additional TKI. For the 59.5% of patients with CP CML treated with 1 prior TKI (imatinib) who achieved a MCyR at any time, the median duration of MCyR was not reached. Among these patients, 65.4% and 42.9% had a MCyR lasting at least 18 and 54 months, respectively. For the 40.2% of patients with CP CML treated with imatinib and at least 1 additional TKI who achieved a MCyR at any time, the median duration of MCyR was not reached. Among these patients, 64.4% and 35.6% had a MCyR lasting at least 9 and 42 months, respectively. Of the 403 treated patients with CP CML, 20 patients had confirmed disease transformation to AP or BP while on treatment with BOSULIF.
The 48-week efficacy results in patients with accelerated and blast phases CML previously treated with at least imatinib are summarized in Table 15.
| AP CML (N=72 evaluable) n (%) | BP CML (N=60 evaluable) n (%) | |
|---|---|---|
| Abbreviations: AP=accelerated phase; BP=blast phase; CHR=complete hematologic response; CI=confidence interval; CML=chronic myelogenous leukemia; CI=confidence interval, OHR=overall hematologic response, CHR=complete hematologic response, N/n=number of patients | ||
| ||
CHR* by Week 48 | 22 (30.6) | 10 (16.7) |
(95% CI) | (20.2, 42.5) | (8.3, 28.5) |
OHR* by Week 48 | 41 (56.9) | 17 (28.3) |
(95% CI) | (44.7, 68.6) | (17.5, 41.4) |
The long-term follow-up data analysis was based on a minimum of 48 months for patients with AP CML and BP CML. Of the 79 treated patients with AP CML, 3 patients had confirmed disease transformation to BP while on BOSULIF treatment.
14.3 Pediatric Patients with Newly-Diagnosed CP Ph+ CML or with CP Ph+ CML with Resistance or Intolerance to Prior Therapy
The efficacy of BOSULIF in pediatric patients with newly-diagnosed (ND) chronic phase (CP) Ph+ CML and patients with resistant/intolerant (R/I) CP Ph+ CML was evaluated in the BCHILD trial [NCT04258943].
The BCHILD trial is a multicenter, non-randomized, open-label study conducted to identify a recommended dose of bosutinib administered orally once daily in pediatric patients with ND CP Ph+ CML and pediatric patients with R/I CP Ph+ CML who have received at least one prior TKI therapy, to estimate the safety and tolerability and efficacy, and to evaluate the PK of bosutinib in this patient population. The study enrolled 28 patients with R/I CP Ph+ CML treated with BOSULIF at 300 mg/m2 to 400 mg/m2 orally once daily, and 21 patients with ND CP Ph+ CML treated at 300 mg/m2 orally once daily. Efficacy outcomes included CCyR (defined as the absence of Ph+ metaphases in chromosome banding analysis of ≥20 metaphases, or <1% BCR-ABL1–positive nuclei of at least 200 peripheral blood interphase nuclei analyzed by Fluorescence In Situ Hybridization (FISH), or MMR if an adequate cytogenetic assessment was unavailable), MCyR (defined as CCyR or partial cytogenetic response of 1% to 35% Ph+ metaphases), and MMR (defined as ≤0.1% BCR-ABL ratio on international scale [IS]) at any time on study.
Patients with ND CP Ph+ CML had a median age of 14 years (range 5 to 17 years); 68% were male; 81% were White, 14% were Black/African American, and 5% were race not reported.
The major (MCyR) and complete (CCyR) cytogenetic responses among patients with ND CP Ph+ CML were 76.2% (95% CI: 52.8, 91.8) and 71.4% (95% CI: 47.8, 88.7), respectively. The MMR among patients with ND CP Ph+ CML was 28.6% (95% CI: 11.3, 52.3). The median duration of follow-up was 14.2 months (range: 1.1, 26.3 months) in patients with ND CP CML.
Patients with R/I CP Ph+ CML included n=6 treated at 300 mg/m2 (0.75 times the recommended dose), n=11 treated at 350 mg/m2 (0.875 times the recommended dose), and n=11 at 400 mg/m2. Overall (n=28), patients had a median age of 11.5 years (range: 1 to 17 years); 57% were male; 43% were White, 7% were Black/African American, 14% were Asian, and 36% were race not reported.
The major (MCyR) and complete (CCyR) cytogenetic responses among patients with R/I CP Ph+ CML were 82.1% (95% CI: 63.1, 93.9) and 78.6% (95% CI: 59.0, 91.7), respectively. The MMR among patients with R/I CP Ph+ CML was 50.0% (95% CI: 30.6, 69.4). The MR4.5 (defined as BCR-ABL/ABL IS ≤ 0.0032%) was 17.9% (95% CI: 6.1, 36.9). Among 14 patients who achieved MMR, two patients lost MMR after 13.6 months and 24.7 months on treatment. The median duration of follow-up for overall survival was 23.2 months (range: 1.0, 61.5 months) in patients with R/I CP Ph+ CML.
How Supplied/Storage and Handling
16 HOW SUPPLIED/STORAGE AND HANDLING
Tablets – How Supplied
BOSULIF (bosutinib) tablets are supplied for oral administration in 3 strengths: a 100 mg yellow, oval, biconvex, film-coated tablet debossed with "Pfizer" on one side and "100" on the other; a 400 mg orange, oval, biconvex, film coated tablet debossed with "Pfizer" on one side and "400" on the other; and a 500 mg red, oval, biconvex, film-coated tablet debossed with "Pfizer" on one side and "500" on the other. BOSULIF (bosutinib) tablets are available in the following packaging configurations with a child-resistant (CR) closure (Table 17) Bottles contain a desiccant.
| BOSULIF Tablets | |||
|---|---|---|---|
| Package Configuration | Tablet Strength (mg) | NDC | Tablet Description |
| Abbreviation: NDC=National drug code. | |||
120 tablets per bottle | 100 mg | 0069-0135-01 | Yellow, oval, biconvex, film-coated tablets, debossed "Pfizer" on one side and "100" on the other. |
30 tablets per bottle | 400 mg | 0069-0193-01 | Orange, oval, biconvex, film-coated tablet debossed with "Pfizer" on one side and "400" on the other. |
30 tablets per bottle | 500 mg | 0069-0136-01 | Red, oval, biconvex, film-coated tablets, debossed "Pfizer" on one side and "500" on the other. |
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Handling and Disposal
Procedures for proper disposal of anticancer drugs should be considered. Touching or handling crushed or broken tablets is to be avoided. Any unused product or waste material should be disposed of in accordance with local requirements, or drug take back programs.
Capsules - How Supplied
BOSULIF (bosutinib) capsules are supplied for oral administration in 2 strengths:
50 mg capsule: size 2 capsule, white body/orange cap with “BOS 50” printed on the body and “Pfizer” printed on the cap in black ink. 100 mg capsule: size 0 capsule, white body/brownish-red cap with “BOS 100” printed on the body and “Pfizer” printed on the cap in black ink. BOSULIF (bosutinib) capsules are available in the following packaging configurations with a CR closure. (Table 18).
| Abbreviation: NDC=National drug code. | |||
BOSULIF Capsules | |||
Package Configuration Count | Capsule Strength (mg) | NDC | Capsule Description |
30 capsules per bottle | 50 | 0069-0504-30 | Size 2 capsule, white body/orange cap with “BOS 50” printed on the body and “Pfizer” printed on the cap in black ink. |
150 capsules per bottle | 100 | 0069-1014-15 | Size 0 capsule, white body/brownish-red cap with “BOS 100” printed on the body and “Pfizer” printed on the cap in black ink. |
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature] and Store and Dispense in Original Container.
Handling and Disposal
Procedures for proper disposal of anticancer drugs should be considered. Patients and/or caregivers must wear gloves while handling the drug product, and wash their hands once finished. Any unused product or waste material should be disposed of in accordance with local requirements.
Medication Guide
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Dosage and Administration
Instruct patients to take BOSULIF exactly as prescribed, not to change their dose or to stop taking BOSULIF unless they are told to do so by their doctor. If patients miss a dose beyond 12 hours, they should be advised to take the next scheduled dose at its regular time. A double dose should not be taken to make up for any missed dose. Advise patients to take BOSULIF with food. Patients should be advised: “Swallow tablets whole. Do not crush, break, or cut tablet. Do not touch or handle crushed or broken tablets.” Patients should be advised: “Capsules may be swallowed whole. For those that cannot swallow the capsule whole, the capsule can be opened and the contents mixed with applesauce or yogurt.”
- •
- Gastrointestinal Toxicity
Advise patients that they may experience diarrhea, nausea, vomiting, abdominal pain, or blood in their stools with BOSULIF and to seek medical attention promptly for these symptoms [see Warnings and Precautions (5.1)].
- •
- Myelosuppression
Advise patients of the possibility of developing low blood cell counts and to immediately report fever, any suggestion of infection, or signs or symptoms suggestive of bleeding or easy bruising [see Warnings and Precautions (5.2)].
- •
- Hepatic Toxicity
Advise patients of the possibility of developing liver function abnormalities and to immediately report jaundice [see Warnings and Precautions (5.3)].
- •
- Cardiovascular Toxicity
Advise patients that cardiac failure, left ventricular dysfunction, and cardiac ischemic events have been reported. Advise patients to seek immediate medical attention if any symptoms suggestive of cardiac failure and cardiac ischemia occur, such as shortness of breath, weight gain, or fluid retention [see Warnings and Precautions (5.4)].
- •
- Fluid Retention
Advise patients of the possibility of developing fluid retention (swelling, weight gain, or shortness of breath) and to seek medical attention promptly if these symptoms arise [see Warnings and Precautions (5.5)].
- •
- Renal Toxicity
Advise patients of the possibility of developing renal problems and to immediately report frequent urination, polyuria or oliguria [see Warnings and Precautions (5.6)].
- •
- Adverse Reactions
Advise patients that they may experience other adverse reactions such as respiratory tract infections, rash, fatigue, loss of appetite, headache, dizziness, back pain, arthralgia, pruritus or constipation with BOSULIF and to seek medical attention if symptoms are significant. There is a possibility of anaphylactic shock [see Contraindications (4) and Adverse Reactions (6)].
- •
- Embryo-Fetal Toxicity
Advise females to inform their healthcare provider if they are pregnant or become pregnant. Advise female patients of the risk to a fetus and potential loss of the pregnancy [see Use in Specific Populations (8.1)].
Advise females of reproductive potential, to use effective contraception during treatment and for 2 weeks after receiving the last dose of BOSULIF [see Warnings and Precautions (5.7) and Use in Specific Populations (8.1, 8.3)].
Advise lactating women not to breastfeed during treatment with BOSULIF and for 2 weeks after the last dose [see Use in Specific Populations (8.2)].
- •
- Drug Interactions
Advise patients that BOSULIF and certain other medicines, including over the counter medications or herbal supplements (such as St. John's wort) can interact with each other and may alter the effects of BOSULIF [see Drug Interactions (7)].
PATIENT INFORMATION | ||
BOSULIF (BAH-su-lif) (bosutinib) tablets | BOSULIF (BAH-su-lif) (bosutinib) capsules | |
What is BOSULIF?
It is not known if BOSULIF is safe and effective in children less than 1 year of age with CP Ph+ CML who are newly‑diagnosed or who no longer benefit from or did not tolerate other treatment or in children with AP Ph+ CML or BP Ph+ CML. | ||
Do not take BOSULIF if you are allergic to bosutinib or any of the ingredients in BOSULIF. See the end of this leaflet for a complete list of ingredients of BOSULIF. | ||
Before taking BOSULIF, tell your doctor about all of your medical conditions, including if you:
Tell your doctor about all the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements. When taken together, BOSULIF and certain other medicines can affect each other. | ||
How should I take BOSULIF?
| ||
What are the possible side effects of BOSULIF?
| ||
|
| |
The most common side effects of BOSULIF in adults and children with CML include: | ||
|
| |
Tell your doctor or get medical help right away if you get respiratory tract infections, loss of appetite, headache, dizziness, back pain, joint pain, rash or itching while taking BOSULIF. These may be symptoms of a severe allergic reaction. | ||
How should I store BOSULIF?
Keep BOSULIF and all medicines out of the reach of children. | ||
General information about the safe and effective use of BOSULIF. | ||
What are the ingredients in BOSULIF? 
| ||
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised 9 2023
Instructions For Use
INSTRUCTIONS FOR USE
BOSULIF (BAH-su-lif)
(bosutinib)
capsules
This Instructions for Use contains information on how to prepare and give a dose of BOSULIF capsules by opening the capsules and mixing the contents with applesauce or yogurt for people who cannot swallow capsules whole. Read this Instructions for Use before you prepare or give the first dose of BOSULIF, and each time you get a refill. Ask your healthcare provider or pharmacist if you have any questions.
Important information you need to know before preparing a dose of BOSULIF capsules:
- •
- BOSULIF capsules can be opened and the capsules contents mixed with room temperature applesauce or yogurt.
- •
- Only use applesauce or yogurt. Do not mix BOSULIF with other foods.
- •
- Swallow all of the mixture right away, without chewing. Do not store the mixture for later use.
- •
- If you do not swallow the entire BOSULIF mixture, do not mix another dose. Wait until the next day to take your regularly scheduled dose.
- •
- Take the BOSULIF mixture with a full meal.
Preparing a dose of BOSULIF capsules:
Gather the following supplies:
- •
- BOSULIF capsules
- •
- small, clean container
- •
- yogurt or applesauce
- •
- teaspoon for mixing
- •
- disposable gloves
Giving a dose of BOSULIF capsules:
Step 1: Choose a clean, flat work surface. Place all supplies on the work surface.
Step 2: Wash and dry your hands well.
Step 3: Put on disposable gloves
Step 4: Get the prescribed number of BOSULIF capsule(s) needed to prepare the dose.
Step 5: Add the amount of applesauce or yogurt needed for the prescribed dose to the container.
Dose | Amount of Applesauce or Yogurt |
100 mg | 10 mL (2 teaspoons) |
150 mg | 15 mL (3 teaspoons) |
200 mg | 20 mL (4 teaspoons) |
250 mg | 25 mL (5 teaspoons) |
300 mg | 30 mL (6 teaspoons) |
350 mg | 30 mL (6 teaspoons) |
400 mg | 35 mL (7 teaspoons) |
450 mg | 40 mL (8 teaspoons) |
500 mg | 45 mL (9 teaspoons) |
550 mg | 45 mL (9 teaspoons) |
600 mg | 50 mL (10 teaspoons) |
Step 6: Carefully open each of the BOSULIF capsule(s) needed for the dose and empty the entire contents into the applesauce or yogurt. Mix the entire capsule contents with the applesauce or yogurt in the container.
Step 7: Swallow all of the mixture right away, without chewing.
Step 8: Dispose of (throw away) the empty BOSULIF capsule shell(s) in the household trash.
Step 9: Wash teaspoon and the container with soap and warm water.
Step 10: Remove disposable gloves and throw them away in the household trash.
Step 11: Wash and dry your hands.
How should I store BOSULIF capsules?
- •
- Store BOSULIF at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Store the BOSULIF capsules in the original bottle.
- •
- Ask your doctor or pharmacist about the right way to throw away outdated or unused BOSULIF.
Keep BOSULIF and all medicines out of the reach of children.
LAB-0639-12.0
For more information, go to www.Bosulif.com or www.pfizermedicalinformation.com or call 1-800-438-1985.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 9 2023
Other
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information. 9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.