DESCRIPTION
Paclitaxel Injection, USP is a clear colorless to slightly yellow viscous solution. It is supplied as a nonaqueous solution intended for dilution with a suitable parenteral fluid prior to intravenous infusion. Paclitaxel is available in 30 mg (5 mL), 100 mg (16.7 mL), and 300 mg (50 mL) multiple-dose vials. Each mL of sterile nonpyrogenic solution contains 6 mg paclitaxel, 527 mg of Polyoxyl 35 Castor Oil, NF, 49.7% (v/v) Dehydrated Alcohol, USP and 2 mg Citric Acid, USP.
Paclitaxel is a natural product with antitumor activity. Paclitaxel is obtained via an extraction process from Taxus X media ‘Hicksii’. The chemical name for paclitaxel is (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4,6,9,11,12,-12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca [3,4] benz [1,2-b] oxet-5-one 6,12b-diacetate, 12-benzoate, 9-ester with (2R,3S)-N-benzoyl-3-phenylisoserine.
Paclitaxel has the following structural formula:
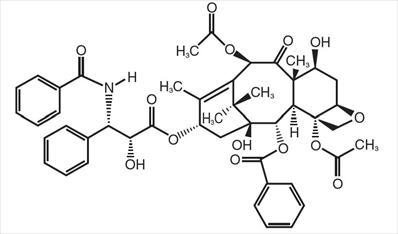
Paclitaxel is a white to off-white crystalline powder with the empirical formula C47H51NO14 and a molecular weight of 853.9. It is highly lipophilic, insoluble in water, and melts at around 216-217°C.