OXBRYTA Instructions For Use
(voxelotor)
This Instructions for Use has been approved by the U.S. Food and Drug Administration. | Revised: 08/2023 | |||
INSTRUCTIONS FOR USE | ||||
This Instructions for Use contains information on how to take OXBRYTA tablets for oral suspension. | ||||
Important Information You Need to Know Before Taking OXBRYTA Tablets for Oral Suspension:
| ||||
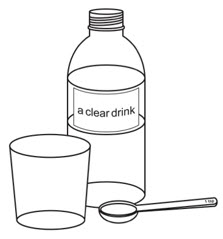
Gather supplies | ||||
You will need the following items to prepare the dose of OXBRYTA tablets for oral suspension (not included with OXBRYTA tablets for oral suspension):
You will also need:
|  | |||
Preparing a dose of OXBRYTA tablets for oral suspension | ||||
Step 1. | Wash and dry your hands well before preparing the dose. | |||
Step 2. | Pour room temperature clear drink into the cup. The table below shows the amount of clear drink needed for your prescribed dose. You may add more clear drink if needed to mix the tablets. | 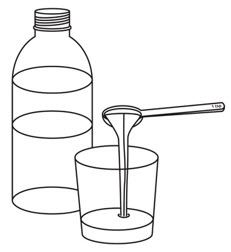 | ||
Number of OXBRYTA Tablets for Oral Suspension | Amount of Clear Drink | |||
1 | 1 teaspoon (5 mL) | |||
2 | 2 teaspoons (10 mL) | |||
3 | 3 teaspoons (15 mL) | |||
4 | 4 teaspoons (20 mL) | |||
5 | 5 teaspoons (25 mL) | |||
7 | 7 teaspoons (35 mL) | |||
8 | 8 teaspoons (40 mL) | |||
| ||||
Step 3. | Add the prescribed number of OXBRYTA tablets for oral suspension into the cup. | 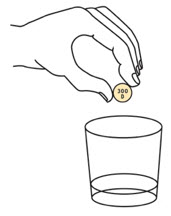 | ||
Step 4. | Swirl the cup until the tablet(s) break apart (disperse) in the drink. Be careful not to spill the mixture.
| 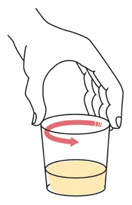 | ||
Step 5. | Wait for 1 to 5 minutes. |  | ||
Giving the dose | ||||
Step 6. | Swirl the cup again. Take or give all of the prepared medicine right away.
| 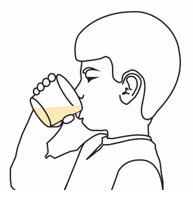 | ||
Step 7. | Add 1 or 2 teaspoons of room temperature clear drink to the cup to make sure the full dose is taken. Swirl the cup until the remaining medicine is mixed and take or give it right away.
| 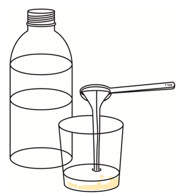 | ||
Step 8. | Wash the teaspoon and cup with warm soap and water. | |||
Storing OXBRYTA
Keep OXBRYTA and all medicines out of the reach of children. | ||||
Disposing of OXBRYTA  Distributed by Global Blood Therapeutics, Inc A subsidiary of Pfizer Inc. South San Francisco, CA 94080 For more information, go to www.pfizer.com or call 1-800-438-1985. | ||||
Find OXBRYTA medical information:
Find OXBRYTA medical information:
OXBRYTA Quick Finder
Health Professional Information
Instructions For Use
This Instructions for Use has been approved by the U.S. Food and Drug Administration. | Revised: 08/2023 | |||
INSTRUCTIONS FOR USE | ||||
This Instructions for Use contains information on how to take OXBRYTA tablets for oral suspension. | ||||
Important Information You Need to Know Before Taking OXBRYTA Tablets for Oral Suspension:
| ||||
Gather supplies | ||||
You will need the following items to prepare the dose of OXBRYTA tablets for oral suspension (not included with OXBRYTA tablets for oral suspension):
You will also need:
|  | |||
Preparing a dose of OXBRYTA tablets for oral suspension | ||||
Step 1. | Wash and dry your hands well before preparing the dose. | |||
Step 2. | Pour room temperature clear drink into the cup. The table below shows the amount of clear drink needed for your prescribed dose. You may add more clear drink if needed to mix the tablets. |  | ||
Number of OXBRYTA Tablets for Oral Suspension | Amount of Clear Drink | |||
1 | 1 teaspoon (5 mL) | |||
2 | 2 teaspoons (10 mL) | |||
3 | 3 teaspoons (15 mL) | |||
4 | 4 teaspoons (20 mL) | |||
5 | 5 teaspoons (25 mL) | |||
7 | 7 teaspoons (35 mL) | |||
8 | 8 teaspoons (40 mL) | |||
| ||||
Step 3. | Add the prescribed number of OXBRYTA tablets for oral suspension into the cup. |  | ||
Step 4. | Swirl the cup until the tablet(s) break apart (disperse) in the drink. Be careful not to spill the mixture.
|  | ||
Step 5. | Wait for 1 to 5 minutes. |  | ||
Giving the dose | ||||
Step 6. | Swirl the cup again. Take or give all of the prepared medicine right away.
|  | ||
Step 7. | Add 1 or 2 teaspoons of room temperature clear drink to the cup to make sure the full dose is taken. Swirl the cup until the remaining medicine is mixed and take or give it right away.
|  | ||
Step 8. | Wash the teaspoon and cup with warm soap and water. | |||
Storing OXBRYTA
Keep OXBRYTA and all medicines out of the reach of children. | ||||
Disposing of OXBRYTA  Distributed by Global Blood Therapeutics, Inc A subsidiary of Pfizer Inc. South San Francisco, CA 94080 For more information, go to www.pfizer.com or call 1-800-438-1985. | ||||
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.