OXBRYTA Clinical Studies
(voxelotor)
14 CLINICAL STUDIES
14.1 Adults and Pediatric Patients 12 Years and Older
The efficacy and safety of OXBRYTA in SCD was evaluated in HOPE, a Phase 3 randomized, double-blind, placebo-controlled, multicenter trial [NCT 03036813]. In this study, 274 patients were randomized to daily oral administration of OXBRYTA 1,500 mg (N=90), OXBRYTA 900 mg (N=92), or placebo (N=92). Patients were included if they had from 1 to 10 vasoocclusive crisis (VOC) events within 12 months prior to enrollment and baseline hemoglobin (Hb) ≥5.5 to ≤10.5 g/dL. Eligible patients on stable doses of hydroxyurea for at least 90 days were allowed to continue hydroxyurea therapy throughout the study. Randomization was stratified by patients already receiving hydroxyurea (yes, no), geographic region (North America, Europe, Other), and age (12 to <17 years, 18 to 65 years). The trial excluded patients who received red blood cell (RBC) transfusions within 60 days and erythropoietin within 28 days of enrollment, had renal insufficiency, uncontrolled liver disease, were pregnant, or lactating.
The majority of patients had HbSS or HbS/beta0-thalassemia genotype (90%) and were receiving background hydroxyurea therapy (65%). The median age was 24 years (range: 12 to 64 years); 46 (17%) patients were 12 to <17 years. Median baseline Hb was 8.5 g/dL (5.9 to 10.8 g/dL). One hundred and fifteen (42%) had 1 VOC event and 159 (58%) had 2 to 10 events within 12 months prior to enrollment. In the OXBRYTA 1,500 mg group, 63 (70%) patients completed the study through Week 72.
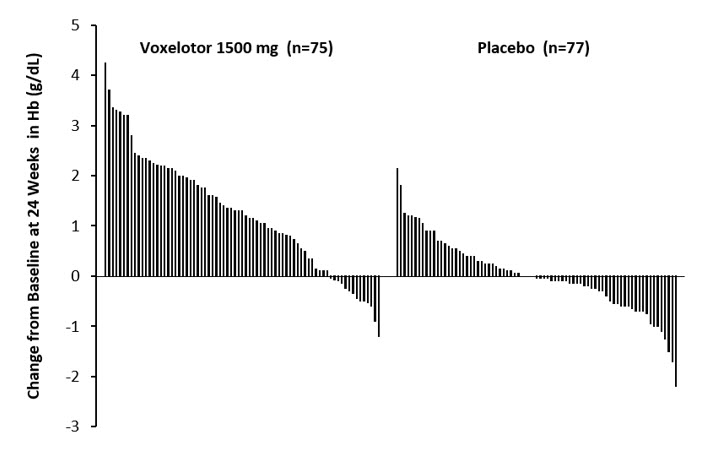
Efficacy was based on Hb response rate defined as a Hb increase of >1 g/dL from baseline to Week 24 in patients treated with OXBRYTA 1,500 mg versus placebo. The response rate for OXBRYTA 1,500 mg was 51.1% (46/90) compared to 6.5% (6/92) in the placebo group (p < 0.001). No outlier subgroups were observed. The distribution of Hb change from baseline for individual patients completing 24 weeks of treatment with OXBRYTA 1,500 mg or placebo is depicted in Figure 1.
|
Figure 1: Subject-level Change from Baseline in Hemoglobin at Week 24 in Patients Who Completed 24 Weeks of Treatment* |
 |
Additional efficacy evaluation included change in Hb and percent change in indirect bilirubin and percent reticulocyte count from baseline to Week 24 (Table 6).
| OXBRYTA 1,500 mg QD (N=90) | Placebo (N=92) | P Value | |
|---|---|---|---|
| QD = once daily; SE = standard error | |||
Hemoglobin | 1.1 g/dL | -0.1 g/dL | < 0.001 |
Indirect Bilirubin | -29.1% | -2.8% | < 0.001 |
Percent Reticulocyte Count | -18.0% | 6.8% | < 0.001 |
14.2 Pediatric Patients 4 to <12 Years
The efficacy and safety of OXBRYTA in patients 4 to <12 years with SCD was evaluated in an open-label, multi-center, Phase 2 trial [NCT 02850406]. In this study, 45 patients 4 to <12 years and 11 patients 12 to <17 years received OXBRYTA. Patients 4 to <12 years received tablets for oral suspension based on body weight at baseline. OXBRYTA doses of 600 mg, 900 mg, or 1,500 mg once daily were administered to patients weighing 10 kg to <20 kg, 20 kg to <40 kg, or ≥40 kg, respectively. Patients 12 to <17 years received OXBRYTA 1,500 mg once daily.
Patients were included if their baseline hemoglobin (Hb) was ≤10.5 g/dL. Eligible patients on stable doses of hydroxyurea for at least 90 days were allowed to continue hydroxyurea therapy throughout the study. The trial excluded patients who had a VOC event within 14 days prior to enrollment, received red blood cell (RBC) transfusions within 30 days of enrollment, and had renal insufficiency or uncontrolled liver disease.
All patients had HbSS or HbS/beta0-thalassemia genotype (100%) and most were receiving background hydroxyurea therapy (80%). The median age was 8 years (range: 4 to 15 years); 45 (80%) patients were 4 to <12 years. In this age group, mean baseline Hb was 8.6 g/dL (range: 6.1 to 10.5 g/dL).
Efficacy was based on Hb response rate, which is defined as a Hb increase of >1 g/dL from baseline to Week 24. Hb response rate for OXBRYTA in patients aged 4 to <12 years who took at least one dose of OXBRYTA was 36% (16/45) (95% CI: 21.6%, 49.5%).
Find OXBRYTA medical information:
Find OXBRYTA medical information:
OXBRYTA Quick Finder
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 Adults and Pediatric Patients 12 Years and Older
The efficacy and safety of OXBRYTA in SCD was evaluated in HOPE, a Phase 3 randomized, double-blind, placebo-controlled, multicenter trial [NCT 03036813]. In this study, 274 patients were randomized to daily oral administration of OXBRYTA 1,500 mg (N=90), OXBRYTA 900 mg (N=92), or placebo (N=92). Patients were included if they had from 1 to 10 vasoocclusive crisis (VOC) events within 12 months prior to enrollment and baseline hemoglobin (Hb) ≥5.5 to ≤10.5 g/dL. Eligible patients on stable doses of hydroxyurea for at least 90 days were allowed to continue hydroxyurea therapy throughout the study. Randomization was stratified by patients already receiving hydroxyurea (yes, no), geographic region (North America, Europe, Other), and age (12 to <17 years, 18 to 65 years). The trial excluded patients who received red blood cell (RBC) transfusions within 60 days and erythropoietin within 28 days of enrollment, had renal insufficiency, uncontrolled liver disease, were pregnant, or lactating.
The majority of patients had HbSS or HbS/beta0-thalassemia genotype (90%) and were receiving background hydroxyurea therapy (65%). The median age was 24 years (range: 12 to 64 years); 46 (17%) patients were 12 to <17 years. Median baseline Hb was 8.5 g/dL (5.9 to 10.8 g/dL). One hundred and fifteen (42%) had 1 VOC event and 159 (58%) had 2 to 10 events within 12 months prior to enrollment. In the OXBRYTA 1,500 mg group, 63 (70%) patients completed the study through Week 72.
Efficacy was based on Hb response rate defined as a Hb increase of >1 g/dL from baseline to Week 24 in patients treated with OXBRYTA 1,500 mg versus placebo. The response rate for OXBRYTA 1,500 mg was 51.1% (46/90) compared to 6.5% (6/92) in the placebo group (p < 0.001). No outlier subgroups were observed. The distribution of Hb change from baseline for individual patients completing 24 weeks of treatment with OXBRYTA 1,500 mg or placebo is depicted in Figure 1.
|
Figure 1: Subject-level Change from Baseline in Hemoglobin at Week 24 in Patients Who Completed 24 Weeks of Treatment* |
 |
Additional efficacy evaluation included change in Hb and percent change in indirect bilirubin and percent reticulocyte count from baseline to Week 24 (Table 6).
| OXBRYTA 1,500 mg QD (N=90) | Placebo (N=92) | P Value | |
|---|---|---|---|
| QD = once daily; SE = standard error | |||
Hemoglobin | 1.1 g/dL | -0.1 g/dL | < 0.001 |
Indirect Bilirubin | -29.1% | -2.8% | < 0.001 |
Percent Reticulocyte Count | -18.0% | 6.8% | < 0.001 |
14.2 Pediatric Patients 4 to <12 Years
The efficacy and safety of OXBRYTA in patients 4 to <12 years with SCD was evaluated in an open-label, multi-center, Phase 2 trial [NCT 02850406]. In this study, 45 patients 4 to <12 years and 11 patients 12 to <17 years received OXBRYTA. Patients 4 to <12 years received tablets for oral suspension based on body weight at baseline. OXBRYTA doses of 600 mg, 900 mg, or 1,500 mg once daily were administered to patients weighing 10 kg to <20 kg, 20 kg to <40 kg, or ≥40 kg, respectively. Patients 12 to <17 years received OXBRYTA 1,500 mg once daily.
Patients were included if their baseline hemoglobin (Hb) was ≤10.5 g/dL. Eligible patients on stable doses of hydroxyurea for at least 90 days were allowed to continue hydroxyurea therapy throughout the study. The trial excluded patients who had a VOC event within 14 days prior to enrollment, received red blood cell (RBC) transfusions within 30 days of enrollment, and had renal insufficiency or uncontrolled liver disease.
All patients had HbSS or HbS/beta0-thalassemia genotype (100%) and most were receiving background hydroxyurea therapy (80%). The median age was 8 years (range: 4 to 15 years); 45 (80%) patients were 4 to <12 years. In this age group, mean baseline Hb was 8.6 g/dL (range: 6.1 to 10.5 g/dL).
Efficacy was based on Hb response rate, which is defined as a Hb increase of >1 g/dL from baseline to Week 24. Hb response rate for OXBRYTA in patients aged 4 to <12 years who took at least one dose of OXBRYTA was 36% (16/45) (95% CI: 21.6%, 49.5%).
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.