oxaliplatin injection Clinical Studies
()
14 CLINICAL STUDIES
14.1 Adjuvant Treatment with Oxaliplatin in Combination with Fluorouracil and Leucovorin
The efficacy of Oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in an international, multicenter, randomized (1:1) trial (The Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer [MOSAIC], NCT00275210) in patients with stage II (Dukes' B2) or III (Dukes' C) colon cancer who had undergone complete resection of the primary tumor. Patients were randomized to receive Oxaliplatin with fluorouracil/leucovorin or fluorouracil/leucovorin alone for a total of 6 months (i.e., 12 cycles). Table 14 shows the dosing regimens for the two arms.
Eligible patients were between 18 and 75 years of age, had histologically proven stage II (T3–T4 N0 M0; Dukes' B2) or III (any T N1–2 M0; Dukes' C) colon carcinoma (with the inferior pole of the tumor above the peritoneal reflection, i.e., greater than or equal to 15 cm from the anal margin) and had undergone (within 7 weeks prior to randomization) complete resection of the primary tumor without gross or microscopic evidence of residual disease and carcinoembryonic antigen (CEA) less than 10 ng/mL. Additional eligibility criteria were no prior chemotherapy, immunotherapy or radiotherapy; Eastern Cooperative Oncology Group performance status of 0, 1, or 2 (Karnofsky Performance Status greater than or equal to 60%); no pre-existing neuropathy; and absolute neutrophil count (ANC) greater than or equal to 1.5 × 109/L, platelets greater than or equal to 100 × 109/L, serum creatinine less than or equal to 1.25 × upper limit normal (ULN), total bilirubin less than 2 × ULN, and aspartate transaminase (AST)/alanine transaminase (ALT) less than 2 × ULN. The major efficacy outcome was 3-year disease-free survival (DFS).
| Treatment Arm | Dose | Regimen |
|---|---|---|
Oxaliplatin + FU/LV | Day 1: Oxaliplatin: 85 mg/m2 (2-hour infusion) + LV: 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks |
FU/LV | Day 1: LV: 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks |
There were 2,246 patients enrolled, of whom 1,347 (60%) had Stage III disease. Tables 15 and 16 show the baseline characteristics and exposure to Oxaliplatin.
| Oxaliplatin + Infusional FU/LV N=1,123 | Infusional FU/LV N=1,123 | ||
|---|---|---|---|
Sex: | Male (%) | 56.1 | 52.4 |
Female (%) | 43.9 | 47.6 | |
Median age (years) | 61.0 | 60.0 | |
<65 years of age (%) | 64.4 | 66.2 | |
≥65 years of age (%) | 35.6 | 33.8 | |
KPS (%) | |||
100 | 29.7 | 30.5 | |
90 | 52.2 | 53.9 | |
80 | 4.4 | 3.3 | |
70 | 13.2 | 11.9 | |
≤60 | 0.6 | 0.4 | |
Primary site (%) | |||
Colon including cecum | 54.6 | 54.4 | |
Sigmoid | 31.9 | 33.8 | |
Recto sigmoid | 12.9 | 10.9 | |
Other including rectum | 0.6 | 0.9 | |
Bowel obstruction (%) | |||
Yes | 17.9 | 19.3 | |
Perforation (%) | |||
Yes | 6.9 | 6.9 | |
Stage at Randomization (%) | |||
II (T=3,4 N=0, M=0) | 40.1 | 39.9 | |
III (T=any, N=1,2, M=0) | 59.6 | 59.3 | |
IV (T=any, N=any, M=1) | 0.4 | 0.8 | |
Staging – T (%) | |||
T1 | 0.5 | 0.7 | |
T2 | 4.5 | 4.8 | |
T3 | 76.0 | 75.9 | |
T4 | 19.0 | 18.5 | |
Staging – N (%) | |||
N0 | 40.2 | 39.9 | |
N1 | 39.4 | 39.4 | |
N2 | 20.4 | 20.7 | |
Staging – M (%) | |||
M1 | 0.4 | 0.8 | |
| Oxaliplatin + Infusional FU/LV N=1,108 | Infusional FU/LV N=1,111 | |
|---|---|---|
Median Relative Dose Intensity (%) | ||
FU | 84.4 | 97.7 |
Oxaliplatin | 80.5 | N/A |
Median Number of Cycles | 12 | 12 |
Median Number of Cycles with Oxaliplatin | 11 | N/A |
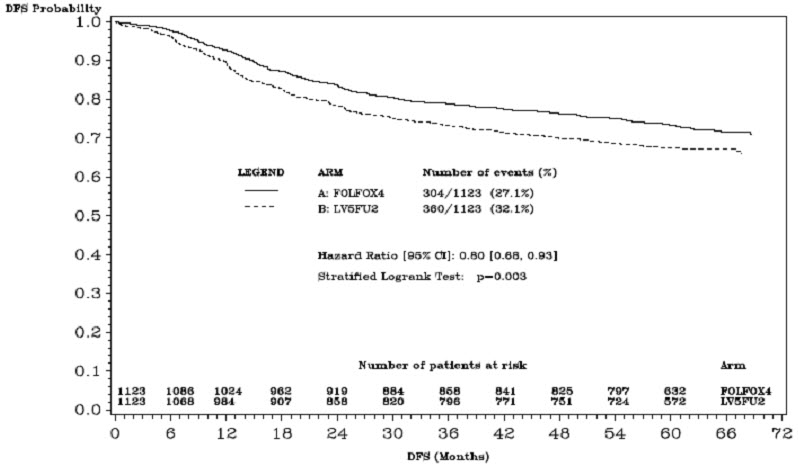
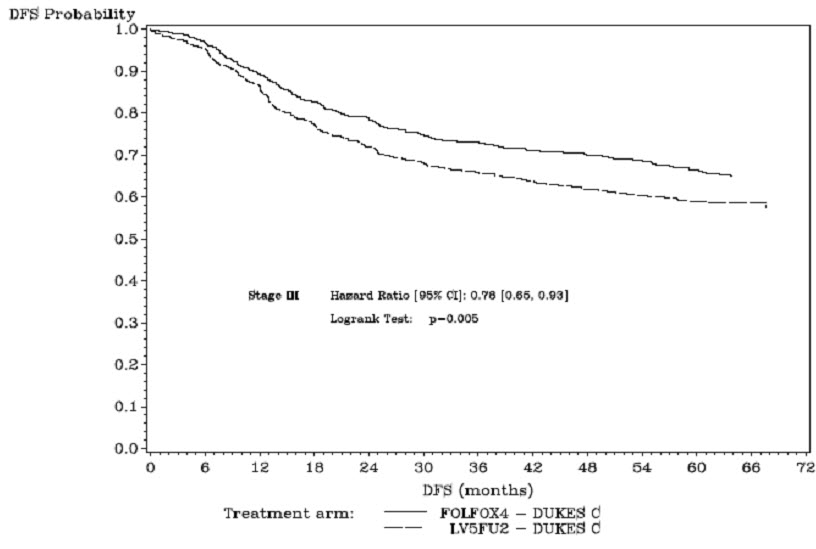
The median duration of follow-up was approximately 77 months. In the overall and the stage III colon cancer populations, DFS was statistically significantly improved in the Oxaliplatin-containing arm compared to fluorouracil/leucovorin alone; however, a statistically significant improvement in DFS was not observed in Stage II patients. No significant differences in overall survival (OS) were detected in the overall population or those with Stage III disease. Table 17 and Figures 1 and 2 summarize the 5-year DFS rates in the overall randomized population and in patients with stage II and III disease based on an intention-to-treat (ITT) analysis.
| Parameter | Oxaliplatin + Infusional FU/LV | Infusional FU/LV |
|---|---|---|
| A hazard ratio of less than 1 favors Oxaliplatin + Infusional FU/LV | ||
| Data cutoff for disease-free survival June 1, 2006 | ||
Overall | ||
Number of patients | 1,123 | 1,123 |
Number of events – relapse or death (%) | 304 (27.1) | 360 (32.1) |
5-yr Disease-free survival % (95% CI) | 73.3 (70.7, 76.0) | 67.4 (64.6, 70.2) |
Hazard ratio (95% CI) | 0.80 (0.68, 0.93) | |
Stratified Log rank test | p=0.003 | |
Stage III (Dukes' C) | ||
Number of patients | 672 | 675 |
Number of events – relapse or death (%) | 226 (33.6) | 271 (40.1) |
5-yr Disease-free survival % (95% CI) | 66.4 (62.7, 70.0) | 58.9 (55.2, 62.7) |
Hazard ratio (95% CI) | 0.78 (0.65, 0.93) | |
Log rank test | p=0.005 | |
Stage II (Dukes' B2) | ||
Number of patients | 451 | 448 |
Number of events – relapse or death (%) | 78 (17.3) | 89 (19.9) |
5-yr Disease-free survival % (95% CI) | 83.7 (80.2, 87.1) | 79.9 (76.2, 83.7) |
Hazard ratio (95% CI) | 0.84 (0.62, 1.14) | |
Log rank test | p=0.258 | |
Figure 1: Kaplan-Meier Curves of Disease-Free Survival (cutoff: 1 June 2006) in Adjuvant Treatment Trial – ITT Population
Figure 2: Kaplan-Meier Curves of Disease-Free Survival in Stage III Patients (cutoff: 1 June 2006) in Adjuvant Treatment Trial – ITT Population
Table 18 summarizes the OS results in the overall randomized population and in patients with stage II and III disease, based on the ITT analysis.
| Parameter | Oxaliplatin + Infusional FU/LV | Infusional FU/LV |
|---|---|---|
| A hazard ratio of less than 1 favors Oxaliplatin + Infusional FU/LV | ||
| Data cut off for overall survival January 16, 2007 | ||
Overall | ||
Number of patients | 1,123 | 1,123 |
Number of death events (%) | 245 (21.8) | 283 (25.2) |
Hazard ratio (95% CI) | 0.84 (0.71, 1.00) | |
Stage III (Dukes' C) | ||
Number of patients | 672 | 675 |
Number of death events (%) | 182 (27.1) | 220 (32.6) |
Hazard ratio (95% CI) | 0.80 (0.65, 0.97) | |
Stage II (Dukes' B2) | ||
Number of patients | 451 | 448 |
Number of death events (%) | 63 (14.0) | 63 (14.1) |
Hazard ratio (95% CI) | 1.00 (0.70, 1.41) | |
14.2 Previously Untreated Advanced Colorectal Cancer
The efficacy of Oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in a North American, multicenter, open-label, randomized, active-controlled trial (A Randomized Phase III Trial of Three Different Regimens of CPT-11 Plus 5-Fluorouracil and Leucovorin Compared to 5-Fluorouracil and Leucovorin in Patients with Advanced Adenocarcinoma of the Colon and Rectum; NCT00003594). The trial included 7 arms at different times during its conduct, four of which were closed due to either changes in the standard of care, toxicity, or simplification. During the trial, the control arm was changed to irinotecan with fluorouracil/leucovorin.
The results reported below compared the efficacy of Oxaliplatin with fluorouracil/leucovorin and Oxaliplatin with irinotecan to an approved control regimen of irinotecan with fluorouracil/leucovorin in 795 concurrently randomized patients previously untreated for locally advanced or metastatic colorectal cancer. Table 19 presents the dosing regimens for the three arms. After completion of enrollment, the dose of irinotecan with fluorouracil/leucovorin was decreased due to toxicity.
Eligible patients were at least 18 years of age; had known locally advanced, locally recurrent, or metastatic colorectal adenocarcinoma not curable by surgery or amenable to radiation therapy; with an Eastern Cooperative Oncology Group (ECOG) performance status ≤0, 1, or 2. Patients had to have absolute neutrophil count (ANC) greater than or equal to 1.5 × 109/L, platelets greater than or equal to 100 × 109/L, hemoglobin greater than or equal to 9.0 g/dL, creatinine less than or equal to 1.5 × upper limit of normal (ULN), total bilirubin less than or equal to 1.5 mg/dL, aspartate transaminase (AST) less than or equal to 5 × ULN, and alkaline phosphatase less than or equal to 5 × ULN. Patients may have received adjuvant treatment for resected Stage II or III disease without recurrence within 12 months. Randomization was stratified by ECOG performance status (0, 1 vs 2), prior adjuvant chemotherapy (yes vs no), prior immunotherapy (yes vs no), and age (less than 65 vs greater than or equal to 65 years). Although no post study treatment was specified in the protocol, 65% to 72% of patients received additional post study chemotherapy after study treatment discontinuation on all arms. Fifty-eight percent of patients on the Oxaliplatin with fluorouracil/leucovorin arm received an irinotecan-containing regimen and 23% of patients on the irinotecan with fluorouracil/leucovorin arm received an oxaliplatin-containing regimen. The main efficacy outcome measure was 3-year disease-free survival (DFS) and additional efficacy outcome measures were overall survival (OS).
| Treatment Arm | Dose | Regimen |
|---|---|---|
Oxaliplatin + FU/LV | Day 1: Oxaliplatin: 85 mg/m2 (2-hour infusion) + LV 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks |
Irinotecan + FU/LV | Day 1: irinotecan 125 mg/m2 as a 90–min infusion + LV 20 mg/m2 as a 15-min infusion or intravenous push, followed by FU 500 mg/m2 intravenous bolus weekly × 4 | every 6 weeks |
Oxaliplatin + Irinotecan | Day 1: Oxaliplatin: 85 mg/m2 intravenous (2-hour infusion) + irinotecan 200 mg/m2 intravenous over 30 minutes | every 3 weeks |
Table 20 presents the baseline characteristics.
| Oxaliplatin + FU/LV N=267 | Irinotecan + FU/LV N=264 | Oxaliplatin + Irinotecan N=264 | ||
|---|---|---|---|---|
Sex: | Male (%) | 58.8 | 65.2 | 61.0 |
Female (%) | 41.2 | 34.8 | 39.0 | |
Median age (years) | 61.0 | 61.0 | 61.0 | |
<65 years of age (%) | 61 | 62 | 63 | |
≥65 years of age (%) | 39 | 38 | 37 | |
ECOG (%) | ||||
0–1 | 94.4 | 95.5 | 94.7 | |
2 | 5.6 | 4.5 | 5.3 | |
Involved organs (%) | ||||
Colon only | 0.7 | 0.8 | 0.4 | |
Liver only | 39.3 | 44.3 | 39.0 | |
Liver + other | 41.2 | 38.6 | 40.9 | |
Lung only | 6.4 | 3.8 | 5.3 | |
Other (including lymph nodes) | 11.6 | 11.0 | 12.9 | |
Not reported | 0.7 | 1.5 | 1.5 | |
Prior radiation (%) | 3.0 | 1.5 | 3.0 | |
Prior surgery (%) | 74.5 | 79.2 | 81.8 | |
Prior adjuvant (%) | 15.7 | 14.8 | 15.2 | |
The median number of cycles administered per patient was 10 (23.9 weeks) for the Oxaliplatin plus fluorouracil/leucovorin regimen, 4 (23.6 weeks) for the irinotecan plus fluorouracil/leucovorin regimen, and 7 (21.0 weeks) for the Oxaliplatin plus irinotecan regimen.
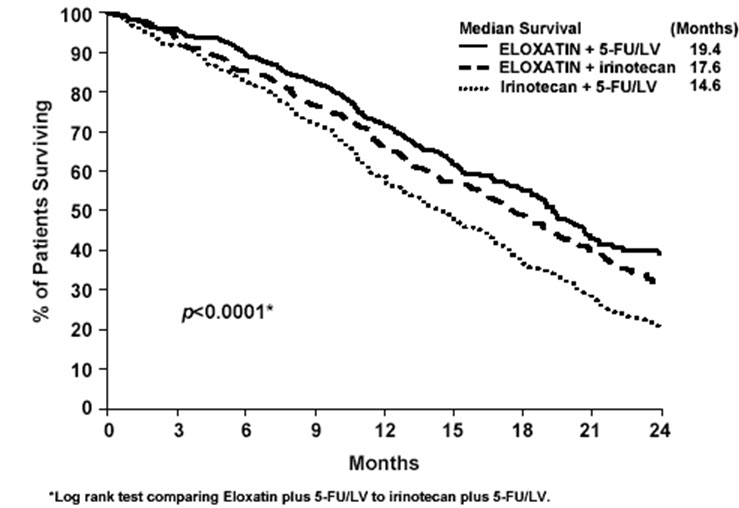
Patients who received Oxaliplatin with fluorouracil/leucovorin had a significantly longer time to tumor progression based on investigator assessment, longer OS, and a significantly higher confirmed response rate based on investigator assessment compared to patients who received irinotecan with fluorouracil/leucovorin. Efficacy results are summarized in Table 21 and Figure 3.
| Oxaliplatin + FU/LV N=267 | Irinotecan + FU/LV N=264 | Oxaliplatin + Irinotecan N=264 | |
|---|---|---|---|
Survival (ITT) | |||
Number of deaths (%) | 155 (58.1) | 192 (72.7) | 175 (66.3) |
Median survival (months) | 19.4 | 14.6 | 17.6 |
Hazard ratio (95% CI)* | 0.65 (0.53, 0.80)† | - | |
P-value | <0.0001† | - | |
TTP (ITT, investigator assessment) | |||
Percentage of progressors | 82.8 | 81.8 | 89.4 |
Median TTP (months) | 8.7 | 6.9 | 6.5 |
Hazard ratio (95% CI)* | 0.74 (0.61, 0.89)† | - | |
P-value | 0.0014† | - | |
Response Rate (investigator assessment)‡ | |||
Patients with measurable disease | 210 | 212 | 215 |
Complete response, N (%) | 13 (6.2) | 5 (2.4) | 7 (3.3) |
Partial response, N (%) | 82 (39.0) | 64 (30.2) | 67 (31.2) |
Complete and partial response, N (%) | 95 (45.2) | 69 (32.5) | 74 (34.4) |
95% CI | (38.5, 52.0) | (26.2, 38.9) | (28.1, 40.8) |
P-value | 0.0080† | - | |
The numbers in the response rate and TTP analysis are based on unblinded investigator assessment.
Figure 3: Kaplan-Meier Curves for Overall Survival in Previously Untreated Advanced Colorectal Cancer Trial
In descriptive subgroup analyses, the improvement in overall survival (OS) for Oxaliplatin with fluorouracil/leucovorin compared to irinotecan with fluorouracil/leucovorin appeared to be maintained across age groups, prior adjuvant treatment, number of organs involved and both sexes; however, the effect appeared larger among women than men.
14.3 Previously Treated Advanced Colorectal Cancer
The efficacy of Oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in a multicenter, open-label, randomized, three-arm controlled trial was conducted in the US and Canada in patients with advanced colorectal cancer who had relapsed/progressed during or within 6 months of first-line treatment with bolus fluorouracil/leucovorin and irinotecan (A multicenter, open-label, randomized, three-arm study of 5-fluorouracil (5-FU) + leucovorin (LV) or oxaliplatin or a combination of 5-FU/LV + oxaliplatin as second-line treatment of metastatic colorectal carcinoma: NCT00008281). Patients were randomized to one of three regimens; the dosing regimens are presented in Table 22. Eligible patients were at least 18 years of age, had unresectable, measurable, histologically proven colorectal adenocarcinoma, with a Karnofsky performance status (KPS) greater than 50%. Patients had to have aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase less than or equal to 2 × upper limit of normal (ULN), unless liver metastases were present and documented at baseline by CT or MRI scan, in which case less than or equal to 5 × ULN was permitted. Prior radiotherapy was permitted if it had been completed at least 3 weeks before randomization. The main efficacy outcome measure was 3-year disease-free survival (DFS) and an additional outcome measure was overall survival (OS).
| Treatment Arm | Dose | Regimen |
|---|---|---|
Oxaliplatin + FU/LV | Day 1: Oxaliplatin: 85 mg/m2 (2-hour infusion) + LV 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks |
FU/LV | Day 1: LV 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks |
Oxaliplatin | Day 1: Oxaliplatin 85 mg/m2 (2-hour infusion) | every 2 weeks |
Patients must have had at least one unidimensional lesion measuring greater than or equal to 20 mm using conventional CT or MRI scans or greater than or equal to 10 mm using a spiral CT scan. Tumor response and progression were assessed every 3 cycles (6 weeks) using the Response Evaluation Criteria in Solid Tumors (RECIST) until radiological documentation of progression or for 13 months following the first dose of study drug(s), whichever came first. Confirmed responses were based on two tumor assessments separated by at least 4 weeks. Baseline characteristics are shown in Table 23.
| Oxaliplatin + FU/LV N=152 | Oxaliplatin N=156 | FU/LV N=151 | ||
|---|---|---|---|---|
Sex: | Male (%) | 57.2 | 60.9 | 54.3 |
Female (%) | 42.8 | 39.1 | 45.7 | |
Median age (years) | 59.0 | 61.0 | 60.0 | |
Range | 22–88 | 27–79 | 21–80 | |
Race (%) | ||||
Caucasian | 88.8 | 84.6 | 87.4 | |
Black | 5.9 | 7.1 | 7.9 | |
Asian | 2.6 | 2.6 | 1.3 | |
Other | 2.6 | 5.8 | 3.3 | |
KPS (%) | ||||
70–100 | 95.4 | 92.3 | 94.7 | |
50–60 | 2.0 | 4.5 | 2.6 | |
Not reported | 2.6 | 3.2 | 2.6 | |
Prior radiotherapy (%) | 25.0 | 19.2 | 25.2 | |
Prior pelvic radiation (%) | 21.1 | 13.5 | 18.5 | |
Number of metastatic sites (%) | ||||
1 | 25.7 | 31.4 | 27.2 | |
≥ 2 | 74.3 | 67.9 | 72.2 | |
Liver involvement (%) | ||||
Liver only | 18.4 | 25.6 | 22.5 | |
Liver + other | 53.3 | 59.0 | 60.3 | |
The median number of cycles administered per patient was 6 for the Oxaliplatin and fluorouracil/leucovorin combination and 3 each for fluorouracil/leucovorin alone and Oxaliplatin alone. Patients treated with the combination of Oxaliplatin and fluorouracil/leucovorin had an increased response rate compared to patients given fluorouracil/leucovorin or oxaliplatin alone. Efficacy results are summarized in Tables 24 and 25.
| Best Response | Oxaliplatin + FU/LV N=152 | Oxaliplatin N=156 | FU/LV N=151 |
|---|---|---|---|
Complete Response | 0 | 0 | 0 |
Partial Response | 13 (9%) | 2 (1%) | 0 |
P-value | 0.0002 FU/LV vs Oxaliplatin + FU/LV | ||
95% CI | 4.6%, 14.2% | 0.2%, 4.6% | 0, 2.4% |
| Arm | Oxaliplatin + FU/LV N=152 | Oxaliplatin N=156 | FU/LV N=151 |
|---|---|---|---|
| |||
Number of progressors | 50 | 101 | 74 |
Number of patients with no radiological evaluation beyond baseline | 17 (11%) | 16 (10%) | 22 (15%) |
Median TTP (months) | 4.6 | 1.6 | 2.7 |
95% CI | 4.2, 6.1 | 1.4, 2.7 | 1.8, 3.0 |
At the time of the interim analysis 49% of the radiographic progression events had occurred. In this interim analysis an estimated 2-month increase in median time to radiographic progression was observed compared to fluorouracil/leucovorin alone.
Find oxaliplatin injection medical information:
Find oxaliplatin injection medical information:
oxaliplatin injection Quick Finder
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 Adjuvant Treatment with Oxaliplatin in Combination with Fluorouracil and Leucovorin
The efficacy of Oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in an international, multicenter, randomized (1:1) trial (The Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer [MOSAIC], NCT00275210) in patients with stage II (Dukes' B2) or III (Dukes' C) colon cancer who had undergone complete resection of the primary tumor. Patients were randomized to receive Oxaliplatin with fluorouracil/leucovorin or fluorouracil/leucovorin alone for a total of 6 months (i.e., 12 cycles). Table 14 shows the dosing regimens for the two arms.
Eligible patients were between 18 and 75 years of age, had histologically proven stage II (T3–T4 N0 M0; Dukes' B2) or III (any T N1–2 M0; Dukes' C) colon carcinoma (with the inferior pole of the tumor above the peritoneal reflection, i.e., greater than or equal to 15 cm from the anal margin) and had undergone (within 7 weeks prior to randomization) complete resection of the primary tumor without gross or microscopic evidence of residual disease and carcinoembryonic antigen (CEA) less than 10 ng/mL. Additional eligibility criteria were no prior chemotherapy, immunotherapy or radiotherapy; Eastern Cooperative Oncology Group performance status of 0, 1, or 2 (Karnofsky Performance Status greater than or equal to 60%); no pre-existing neuropathy; and absolute neutrophil count (ANC) greater than or equal to 1.5 × 109/L, platelets greater than or equal to 100 × 109/L, serum creatinine less than or equal to 1.25 × upper limit normal (ULN), total bilirubin less than 2 × ULN, and aspartate transaminase (AST)/alanine transaminase (ALT) less than 2 × ULN. The major efficacy outcome was 3-year disease-free survival (DFS).
| Treatment Arm | Dose | Regimen |
|---|---|---|
Oxaliplatin + FU/LV | Day 1: Oxaliplatin: 85 mg/m2 (2-hour infusion) + LV: 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks |
FU/LV | Day 1: LV: 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks |
There were 2,246 patients enrolled, of whom 1,347 (60%) had Stage III disease. Tables 15 and 16 show the baseline characteristics and exposure to Oxaliplatin.
| Oxaliplatin + Infusional FU/LV N=1,123 | Infusional FU/LV N=1,123 | ||
|---|---|---|---|
Sex: | Male (%) | 56.1 | 52.4 |
Female (%) | 43.9 | 47.6 | |
Median age (years) | 61.0 | 60.0 | |
<65 years of age (%) | 64.4 | 66.2 | |
≥65 years of age (%) | 35.6 | 33.8 | |
KPS (%) | |||
100 | 29.7 | 30.5 | |
90 | 52.2 | 53.9 | |
80 | 4.4 | 3.3 | |
70 | 13.2 | 11.9 | |
≤60 | 0.6 | 0.4 | |
Primary site (%) | |||
Colon including cecum | 54.6 | 54.4 | |
Sigmoid | 31.9 | 33.8 | |
Recto sigmoid | 12.9 | 10.9 | |
Other including rectum | 0.6 | 0.9 | |
Bowel obstruction (%) | |||
Yes | 17.9 | 19.3 | |
Perforation (%) | |||
Yes | 6.9 | 6.9 | |
Stage at Randomization (%) | |||
II (T=3,4 N=0, M=0) | 40.1 | 39.9 | |
III (T=any, N=1,2, M=0) | 59.6 | 59.3 | |
IV (T=any, N=any, M=1) | 0.4 | 0.8 | |
Staging – T (%) | |||
T1 | 0.5 | 0.7 | |
T2 | 4.5 | 4.8 | |
T3 | 76.0 | 75.9 | |
T4 | 19.0 | 18.5 | |
Staging – N (%) | |||
N0 | 40.2 | 39.9 | |
N1 | 39.4 | 39.4 | |
N2 | 20.4 | 20.7 | |
Staging – M (%) | |||
M1 | 0.4 | 0.8 | |
| Oxaliplatin + Infusional FU/LV N=1,108 | Infusional FU/LV N=1,111 | |
|---|---|---|
Median Relative Dose Intensity (%) | ||
FU | 84.4 | 97.7 |
Oxaliplatin | 80.5 | N/A |
Median Number of Cycles | 12 | 12 |
Median Number of Cycles with Oxaliplatin | 11 | N/A |
The median duration of follow-up was approximately 77 months. In the overall and the stage III colon cancer populations, DFS was statistically significantly improved in the Oxaliplatin-containing arm compared to fluorouracil/leucovorin alone; however, a statistically significant improvement in DFS was not observed in Stage II patients. No significant differences in overall survival (OS) were detected in the overall population or those with Stage III disease. Table 17 and Figures 1 and 2 summarize the 5-year DFS rates in the overall randomized population and in patients with stage II and III disease based on an intention-to-treat (ITT) analysis.
| Parameter | Oxaliplatin + Infusional FU/LV | Infusional FU/LV |
|---|---|---|
| A hazard ratio of less than 1 favors Oxaliplatin + Infusional FU/LV | ||
| Data cutoff for disease-free survival June 1, 2006 | ||
Overall | ||
Number of patients | 1,123 | 1,123 |
Number of events – relapse or death (%) | 304 (27.1) | 360 (32.1) |
5-yr Disease-free survival % (95% CI) | 73.3 (70.7, 76.0) | 67.4 (64.6, 70.2) |
Hazard ratio (95% CI) | 0.80 (0.68, 0.93) | |
Stratified Log rank test | p=0.003 | |
Stage III (Dukes' C) | ||
Number of patients | 672 | 675 |
Number of events – relapse or death (%) | 226 (33.6) | 271 (40.1) |
5-yr Disease-free survival % (95% CI) | 66.4 (62.7, 70.0) | 58.9 (55.2, 62.7) |
Hazard ratio (95% CI) | 0.78 (0.65, 0.93) | |
Log rank test | p=0.005 | |
Stage II (Dukes' B2) | ||
Number of patients | 451 | 448 |
Number of events – relapse or death (%) | 78 (17.3) | 89 (19.9) |
5-yr Disease-free survival % (95% CI) | 83.7 (80.2, 87.1) | 79.9 (76.2, 83.7) |
Hazard ratio (95% CI) | 0.84 (0.62, 1.14) | |
Log rank test | p=0.258 | |
Figure 1: Kaplan-Meier Curves of Disease-Free Survival (cutoff: 1 June 2006) in Adjuvant Treatment Trial – ITT Population
Figure 2: Kaplan-Meier Curves of Disease-Free Survival in Stage III Patients (cutoff: 1 June 2006) in Adjuvant Treatment Trial – ITT Population
Table 18 summarizes the OS results in the overall randomized population and in patients with stage II and III disease, based on the ITT analysis.
| Parameter | Oxaliplatin + Infusional FU/LV | Infusional FU/LV |
|---|---|---|
| A hazard ratio of less than 1 favors Oxaliplatin + Infusional FU/LV | ||
| Data cut off for overall survival January 16, 2007 | ||
Overall | ||
Number of patients | 1,123 | 1,123 |
Number of death events (%) | 245 (21.8) | 283 (25.2) |
Hazard ratio (95% CI) | 0.84 (0.71, 1.00) | |
Stage III (Dukes' C) | ||
Number of patients | 672 | 675 |
Number of death events (%) | 182 (27.1) | 220 (32.6) |
Hazard ratio (95% CI) | 0.80 (0.65, 0.97) | |
Stage II (Dukes' B2) | ||
Number of patients | 451 | 448 |
Number of death events (%) | 63 (14.0) | 63 (14.1) |
Hazard ratio (95% CI) | 1.00 (0.70, 1.41) | |
14.2 Previously Untreated Advanced Colorectal Cancer
The efficacy of Oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in a North American, multicenter, open-label, randomized, active-controlled trial (A Randomized Phase III Trial of Three Different Regimens of CPT-11 Plus 5-Fluorouracil and Leucovorin Compared to 5-Fluorouracil and Leucovorin in Patients with Advanced Adenocarcinoma of the Colon and Rectum; NCT00003594). The trial included 7 arms at different times during its conduct, four of which were closed due to either changes in the standard of care, toxicity, or simplification. During the trial, the control arm was changed to irinotecan with fluorouracil/leucovorin.
The results reported below compared the efficacy of Oxaliplatin with fluorouracil/leucovorin and Oxaliplatin with irinotecan to an approved control regimen of irinotecan with fluorouracil/leucovorin in 795 concurrently randomized patients previously untreated for locally advanced or metastatic colorectal cancer. Table 19 presents the dosing regimens for the three arms. After completion of enrollment, the dose of irinotecan with fluorouracil/leucovorin was decreased due to toxicity.
Eligible patients were at least 18 years of age; had known locally advanced, locally recurrent, or metastatic colorectal adenocarcinoma not curable by surgery or amenable to radiation therapy; with an Eastern Cooperative Oncology Group (ECOG) performance status ≤0, 1, or 2. Patients had to have absolute neutrophil count (ANC) greater than or equal to 1.5 × 109/L, platelets greater than or equal to 100 × 109/L, hemoglobin greater than or equal to 9.0 g/dL, creatinine less than or equal to 1.5 × upper limit of normal (ULN), total bilirubin less than or equal to 1.5 mg/dL, aspartate transaminase (AST) less than or equal to 5 × ULN, and alkaline phosphatase less than or equal to 5 × ULN. Patients may have received adjuvant treatment for resected Stage II or III disease without recurrence within 12 months. Randomization was stratified by ECOG performance status (0, 1 vs 2), prior adjuvant chemotherapy (yes vs no), prior immunotherapy (yes vs no), and age (less than 65 vs greater than or equal to 65 years). Although no post study treatment was specified in the protocol, 65% to 72% of patients received additional post study chemotherapy after study treatment discontinuation on all arms. Fifty-eight percent of patients on the Oxaliplatin with fluorouracil/leucovorin arm received an irinotecan-containing regimen and 23% of patients on the irinotecan with fluorouracil/leucovorin arm received an oxaliplatin-containing regimen. The main efficacy outcome measure was 3-year disease-free survival (DFS) and additional efficacy outcome measures were overall survival (OS).
| Treatment Arm | Dose | Regimen |
|---|---|---|
Oxaliplatin + FU/LV | Day 1: Oxaliplatin: 85 mg/m2 (2-hour infusion) + LV 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks |
Irinotecan + FU/LV | Day 1: irinotecan 125 mg/m2 as a 90–min infusion + LV 20 mg/m2 as a 15-min infusion or intravenous push, followed by FU 500 mg/m2 intravenous bolus weekly × 4 | every 6 weeks |
Oxaliplatin + Irinotecan | Day 1: Oxaliplatin: 85 mg/m2 intravenous (2-hour infusion) + irinotecan 200 mg/m2 intravenous over 30 minutes | every 3 weeks |
Table 20 presents the baseline characteristics.
| Oxaliplatin + FU/LV N=267 | Irinotecan + FU/LV N=264 | Oxaliplatin + Irinotecan N=264 | ||
|---|---|---|---|---|
Sex: | Male (%) | 58.8 | 65.2 | 61.0 |
Female (%) | 41.2 | 34.8 | 39.0 | |
Median age (years) | 61.0 | 61.0 | 61.0 | |
<65 years of age (%) | 61 | 62 | 63 | |
≥65 years of age (%) | 39 | 38 | 37 | |
ECOG (%) | ||||
0–1 | 94.4 | 95.5 | 94.7 | |
2 | 5.6 | 4.5 | 5.3 | |
Involved organs (%) | ||||
Colon only | 0.7 | 0.8 | 0.4 | |
Liver only | 39.3 | 44.3 | 39.0 | |
Liver + other | 41.2 | 38.6 | 40.9 | |
Lung only | 6.4 | 3.8 | 5.3 | |
Other (including lymph nodes) | 11.6 | 11.0 | 12.9 | |
Not reported | 0.7 | 1.5 | 1.5 | |
Prior radiation (%) | 3.0 | 1.5 | 3.0 | |
Prior surgery (%) | 74.5 | 79.2 | 81.8 | |
Prior adjuvant (%) | 15.7 | 14.8 | 15.2 | |
The median number of cycles administered per patient was 10 (23.9 weeks) for the Oxaliplatin plus fluorouracil/leucovorin regimen, 4 (23.6 weeks) for the irinotecan plus fluorouracil/leucovorin regimen, and 7 (21.0 weeks) for the Oxaliplatin plus irinotecan regimen.
Patients who received Oxaliplatin with fluorouracil/leucovorin had a significantly longer time to tumor progression based on investigator assessment, longer OS, and a significantly higher confirmed response rate based on investigator assessment compared to patients who received irinotecan with fluorouracil/leucovorin. Efficacy results are summarized in Table 21 and Figure 3.
| Oxaliplatin + FU/LV N=267 | Irinotecan + FU/LV N=264 | Oxaliplatin + Irinotecan N=264 | |
|---|---|---|---|
Survival (ITT) | |||
Number of deaths (%) | 155 (58.1) | 192 (72.7) | 175 (66.3) |
Median survival (months) | 19.4 | 14.6 | 17.6 |
Hazard ratio (95% CI)* | 0.65 (0.53, 0.80)† | - | |
P-value | <0.0001† | - | |
TTP (ITT, investigator assessment) | |||
Percentage of progressors | 82.8 | 81.8 | 89.4 |
Median TTP (months) | 8.7 | 6.9 | 6.5 |
Hazard ratio (95% CI)* | 0.74 (0.61, 0.89)† | - | |
P-value | 0.0014† | - | |
Response Rate (investigator assessment)‡ | |||
Patients with measurable disease | 210 | 212 | 215 |
Complete response, N (%) | 13 (6.2) | 5 (2.4) | 7 (3.3) |
Partial response, N (%) | 82 (39.0) | 64 (30.2) | 67 (31.2) |
Complete and partial response, N (%) | 95 (45.2) | 69 (32.5) | 74 (34.4) |
95% CI | (38.5, 52.0) | (26.2, 38.9) | (28.1, 40.8) |
P-value | 0.0080† | - | |
The numbers in the response rate and TTP analysis are based on unblinded investigator assessment.
Figure 3: Kaplan-Meier Curves for Overall Survival in Previously Untreated Advanced Colorectal Cancer Trial
In descriptive subgroup analyses, the improvement in overall survival (OS) for Oxaliplatin with fluorouracil/leucovorin compared to irinotecan with fluorouracil/leucovorin appeared to be maintained across age groups, prior adjuvant treatment, number of organs involved and both sexes; however, the effect appeared larger among women than men.
14.3 Previously Treated Advanced Colorectal Cancer
The efficacy of Oxaliplatin in combination with fluorouracil (FU)/leucovorin (LV) was evaluated in a multicenter, open-label, randomized, three-arm controlled trial was conducted in the US and Canada in patients with advanced colorectal cancer who had relapsed/progressed during or within 6 months of first-line treatment with bolus fluorouracil/leucovorin and irinotecan (A multicenter, open-label, randomized, three-arm study of 5-fluorouracil (5-FU) + leucovorin (LV) or oxaliplatin or a combination of 5-FU/LV + oxaliplatin as second-line treatment of metastatic colorectal carcinoma: NCT00008281). Patients were randomized to one of three regimens; the dosing regimens are presented in Table 22. Eligible patients were at least 18 years of age, had unresectable, measurable, histologically proven colorectal adenocarcinoma, with a Karnofsky performance status (KPS) greater than 50%. Patients had to have aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase less than or equal to 2 × upper limit of normal (ULN), unless liver metastases were present and documented at baseline by CT or MRI scan, in which case less than or equal to 5 × ULN was permitted. Prior radiotherapy was permitted if it had been completed at least 3 weeks before randomization. The main efficacy outcome measure was 3-year disease-free survival (DFS) and an additional outcome measure was overall survival (OS).
| Treatment Arm | Dose | Regimen |
|---|---|---|
Oxaliplatin + FU/LV | Day 1: Oxaliplatin: 85 mg/m2 (2-hour infusion) + LV 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks |
FU/LV | Day 1: LV 200 mg/m2 (2-hour infusion), followed by FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks |
Oxaliplatin | Day 1: Oxaliplatin 85 mg/m2 (2-hour infusion) | every 2 weeks |
Patients must have had at least one unidimensional lesion measuring greater than or equal to 20 mm using conventional CT or MRI scans or greater than or equal to 10 mm using a spiral CT scan. Tumor response and progression were assessed every 3 cycles (6 weeks) using the Response Evaluation Criteria in Solid Tumors (RECIST) until radiological documentation of progression or for 13 months following the first dose of study drug(s), whichever came first. Confirmed responses were based on two tumor assessments separated by at least 4 weeks. Baseline characteristics are shown in Table 23.
| Oxaliplatin + FU/LV N=152 | Oxaliplatin N=156 | FU/LV N=151 | ||
|---|---|---|---|---|
Sex: | Male (%) | 57.2 | 60.9 | 54.3 |
Female (%) | 42.8 | 39.1 | 45.7 | |
Median age (years) | 59.0 | 61.0 | 60.0 | |
Range | 22–88 | 27–79 | 21–80 | |
Race (%) | ||||
Caucasian | 88.8 | 84.6 | 87.4 | |
Black | 5.9 | 7.1 | 7.9 | |
Asian | 2.6 | 2.6 | 1.3 | |
Other | 2.6 | 5.8 | 3.3 | |
KPS (%) | ||||
70–100 | 95.4 | 92.3 | 94.7 | |
50–60 | 2.0 | 4.5 | 2.6 | |
Not reported | 2.6 | 3.2 | 2.6 | |
Prior radiotherapy (%) | 25.0 | 19.2 | 25.2 | |
Prior pelvic radiation (%) | 21.1 | 13.5 | 18.5 | |
Number of metastatic sites (%) | ||||
1 | 25.7 | 31.4 | 27.2 | |
≥ 2 | 74.3 | 67.9 | 72.2 | |
Liver involvement (%) | ||||
Liver only | 18.4 | 25.6 | 22.5 | |
Liver + other | 53.3 | 59.0 | 60.3 | |
The median number of cycles administered per patient was 6 for the Oxaliplatin and fluorouracil/leucovorin combination and 3 each for fluorouracil/leucovorin alone and Oxaliplatin alone. Patients treated with the combination of Oxaliplatin and fluorouracil/leucovorin had an increased response rate compared to patients given fluorouracil/leucovorin or oxaliplatin alone. Efficacy results are summarized in Tables 24 and 25.
| Best Response | Oxaliplatin + FU/LV N=152 | Oxaliplatin N=156 | FU/LV N=151 |
|---|---|---|---|
Complete Response | 0 | 0 | 0 |
Partial Response | 13 (9%) | 2 (1%) | 0 |
P-value | 0.0002 FU/LV vs Oxaliplatin + FU/LV | ||
95% CI | 4.6%, 14.2% | 0.2%, 4.6% | 0, 2.4% |
| Arm | Oxaliplatin + FU/LV N=152 | Oxaliplatin N=156 | FU/LV N=151 |
|---|---|---|---|
| |||
Number of progressors | 50 | 101 | 74 |
Number of patients with no radiological evaluation beyond baseline | 17 (11%) | 16 (10%) | 22 (15%) |
Median TTP (months) | 4.6 | 1.6 | 2.7 |
95% CI | 4.2, 6.1 | 1.4, 2.7 | 1.8, 3.0 |
At the time of the interim analysis 49% of the radiographic progression events had occurred. In this interim analysis an estimated 2-month increase in median time to radiographic progression was observed compared to fluorouracil/leucovorin alone.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.