DESCRIPTION
Metoclopramide hydrochloride is a white or practically white, crystalline, odorless or practically odorless powder. It is very soluble in water, freely soluble in alcohol, sparingly soluble in chloroform, practically insoluble in ether. Chemically it is 4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxybenzamide monohydrochloride monohydrate. It has the following structural formula:
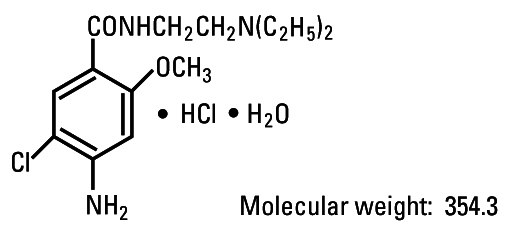
Molecular formula: C14H22ClN3O2 • HCl • H2O
Metoclopramide Injection, USP is a sterile, nonpyrogenic solution of metoclopramide hydrochloride in water for injection. Each milliliter contains metoclopramide base 5 mg (as the hydrochloride monohydrate); 8.5 mg sodium chloride. May contain hydrochloric acid and/or sodium hydroxide for pH adjustment; pH 4.4 (2.5 to 6.5).
The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended for use only as a single-dose injection. When smaller doses are required, the unused portion should be discarded.
This product is light sensitive. It should be inspected before use and discarded if either color or particulate is observed.
Metoclopramide Injection is intended for intravenous or intramuscular administration.