MEKTOVI® Clinical Studies
(binimetinib)
14 CLINICAL STUDIES
14.1 BRAF V600E or V600K Mutation-Positive Unresectable or Metastatic Melanoma
MEKTOVI in combination with encorafenib was evaluated in a randomized, active-controlled, open-label, multicenter trial (COLUMBUS; NCT01909453). Eligible patients were required to have BRAF V600E or V600K mutation-positive unresectable or metastatic melanoma, as detected using the bioMerieux THxID™BRAF assay. Patients were permitted to have received immunotherapy in the adjuvant setting and one prior line of immunotherapy for unresectable locally advanced or metastatic disease. Prior use of BRAF inhibitors or MEK inhibitors was prohibited. Randomization was stratified by American Joint Committee on Cancer (AJCC) Stage (IIIB, IIIC, IVM1a or IVM1b, versus IVM1c), Eastern Cooperative Oncology Group (ECOG) performance status (0 versus 1), and prior immunotherapy for unresectable or metastatic disease (yes versus no).
Patients were randomized (1:1:1) to receive MEKTOVI 45 mg twice daily in combination with encorafenib 450 mg once daily (MEKTOVI in combination with encorafenib), encorafenib 300 mg once daily, or vemurafenib 960 mg twice daily. Treatment continued until disease progression or unacceptable toxicity. Only the results of the approved dosing (MEKTOVI 45 mg in combination with encorafenib 450 mg) are described below.
The major efficacy outcome measure was progression-free survival (PFS), as assessed by a blinded independent central review, to compare MEKTOVI in combination with encorafenib with vemurafenib. Additional efficacy measures included overall survival (OS), as well as objective response rate (ORR) and duration of response (DoR) which were assessed by central review.
A total of 577 patients were randomized, 192 to the MEKTOVI in combination with encorafenib arm, 194 to the encorafenib arm, and 191 to the vemurafenib arm. Of the 383 patients randomized to either the MEKTOVI in combination with encorafenib or the vemurafenib arms, the median age was 56 years (20 to 89 years), 59% were male, 91% were White, and 72% had baseline ECOG performance status of 0. Ninety-five percent (95%) had metastatic disease, 65% were Stage IVM1c, and 4% received prior CTLA-4, PD-1, or PD-L1 directed antibodies. Twenty-eight percent (28%) had elevated baseline serum lactate dehydrogenase (LDH), 45% had ≥3 organs with tumor involvement at baseline, and 3% had brain metastases. Based on centralized testing, 100% of patients’ tumors tested positive for BRAF mutations; BRAF V600E (88%), BRAF V600K (11%), or both (<1%).
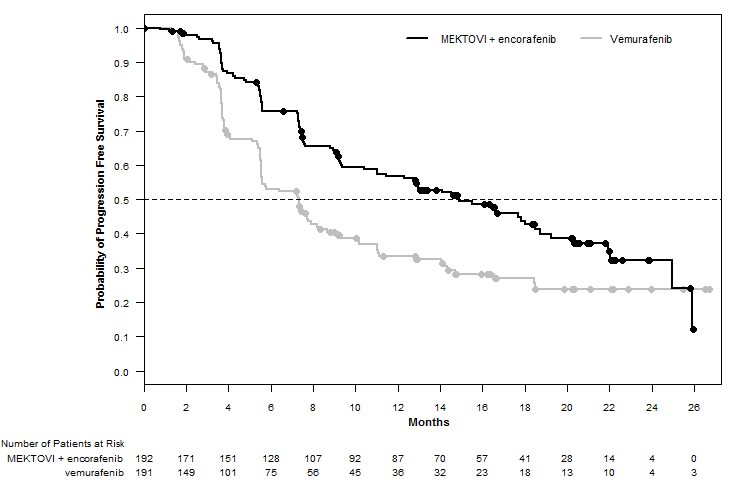
MEKTOVI in combination with encorafenib demonstrated a statistically significant improvement in PFS compared to vemurafenib. Efficacy results are summarized in Table 7 and Figure 1.
| CI = Confidence interval; CR = Complete response; DoR = Duration of response; HR = Hazard ratio; NE = Not estimable; ORR = Overall response rate; OS = Overall survival; PFS = Progression-free survival; PR = Partial response. | ||
| ||
MEKTOVI with encorafenib N=192 | Vemurafenib N=191 | |
Progression-Free Survival | ||
Number of events (%) | 98 (51) | 106 (55) |
Progressive disease | 88 (46) | 104 (54) |
Death | 10 (5) | 2 (1) |
Median PFS, months (95% CI) | 14.9 (11, 18.5) | 7.3 (5.6, 8.2) |
HR (95% CI)* | 0.54 (0.41, 0.71) | |
P value† | <0.0001 | |
Overall Survival‡ | ||
Number of events (%) | 105 (55) | 127 (67) |
Median OS, months (95% CI) | 33.6 (22.4, 39.2) | 16.9 (14.0, 24.5) |
HR (95% CI) * | 0.61 (0.47, 0.79) | |
Overall Response Rate | ||
ORR (95% CI) | 63% (56%, 70%) | 40% (33%, 48%) |
CR | 8% | 6% |
PR | 55% | 35% |
Duration of Response | ||
Median DoR, months (95% CI) | 16.6 (12.2, 20.4) | 12.3 (6.9, 16.9) |
Figure 1: Kaplan-Meier Curves for Progression-Free Survival in COLUMBUS
14.2 BRAF V600E Mutation-Positive Metastatic Non-Small Cell Lung Cancer
MEKTOVI in combination with encorafenib was evaluated in an open-label, multicenter, single-arm study in patients with BRAF V600E mutation-positive metastatic non-small cell lung cancer (NSCLC) (PHAROS; NCT03915951). Eligible patients had a diagnosis of histologically-confirmed metastatic NSCLC with BRAF V600E mutation that was treatment-naïve or had been previously treated with 1 prior line of systemic therapy in the metastatic setting (platinum-based chemotherapy and/or anti-PD-1/PD-L1 therapies), age 18 years or older, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, and measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Prior use of BRAF inhibitors or MEK inhibitors was not allowed.
Patients received MEKTOVI 45 mg orally twice daily and encorafenib 450 mg once daily until disease progression or unacceptable toxicity. The major efficacy outcome measures were objective response rate (ORR) per RECIST v1.1 and duration of response (DoR) as assessed by independent review committee (IRC).
In the efficacy population, BRAF V600E mutation status was determined by prospective local testing using tumor tissue (78%) or blood (22%) specimens. Of the 98 patients with BRAF V600E mutation, 6 patients were enrolled into the trial based on testing of their tumor tissue specimens with the FoundationOne CDx tissue test. Of the remaining 92 patients enrolled based on local testing, 68 patients had their tumor tissue specimens retrospectively confirmed as having BRAF V600E positive status by the FoundationOne CDx tissue test. The remaining patients had either BRAF V600E negative status (n=5) or had unevaluable results (n=19) by the FoundationOne CDx tissue test. In addition, plasma samples from 81 out of 98 patients were retrospectively tested using the FoundationOne Liquid CDx assay. Of the 81 patients, 48 were confirmed positive for BRAF V600E, while 33 patients were BRAF V600E mutation negative by FoundationOne Liquid CDx assay. The remaining 17 samples had unevaluable results with FoundationOne Liquid CDx assay.
The efficacy population included 59 treatment-naïve patients and 39 previously-treated patients. Among these 98 patients, the median age was 70 years (range: 47 to 86); 53% female; 88% White, 7% Asian, 3% Black or African American, and 1% American Indian or Alaska Native; 99% were not Hispanic or Latino; 13% were current smokers and 57% were former smokers; 73% had ECOG PS of 1; and 97% had adenocarcinoma. All patients had metastatic disease and 8% had brain metastases at baseline.
Efficacy results for patients with BRAF V600E mutation-positive metastatic NSCLC are summarized in Table 8.
| CI = Confidence interval; CR = Complete response; DoR = Duration of response; N = Number of patients; NE = Not estimable; ORR = Objective response rate; PR = Partial response. | ||
| ||
MEKTOVI with encorafenib | ||
Efficacy Parameter | Treatment naïve (N=59) | Previously treated (N=39) |
Objective Response Rate* | ||
ORR (95% CI) | 75% (62, 85) | 46% (30, 63) |
CR | 15% | 10% |
PR | 59% | 36% |
Duration of Response* | N=44 | N=18 |
Median DoR, months | NE (23.1, NE) | 16.7 (7.4, NE) |
% with DoR ≥6 months | 75% | 67% |
% with DoR ≥12 months | 59% | 33% |
Find MEKTOVI® medical information:
Find MEKTOVI® medical information:
MEKTOVI® Quick Finder
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 BRAF V600E or V600K Mutation-Positive Unresectable or Metastatic Melanoma
MEKTOVI in combination with encorafenib was evaluated in a randomized, active-controlled, open-label, multicenter trial (COLUMBUS; NCT01909453). Eligible patients were required to have BRAF V600E or V600K mutation-positive unresectable or metastatic melanoma, as detected using the bioMerieux THxID™BRAF assay. Patients were permitted to have received immunotherapy in the adjuvant setting and one prior line of immunotherapy for unresectable locally advanced or metastatic disease. Prior use of BRAF inhibitors or MEK inhibitors was prohibited. Randomization was stratified by American Joint Committee on Cancer (AJCC) Stage (IIIB, IIIC, IVM1a or IVM1b, versus IVM1c), Eastern Cooperative Oncology Group (ECOG) performance status (0 versus 1), and prior immunotherapy for unresectable or metastatic disease (yes versus no).
Patients were randomized (1:1:1) to receive MEKTOVI 45 mg twice daily in combination with encorafenib 450 mg once daily (MEKTOVI in combination with encorafenib), encorafenib 300 mg once daily, or vemurafenib 960 mg twice daily. Treatment continued until disease progression or unacceptable toxicity. Only the results of the approved dosing (MEKTOVI 45 mg in combination with encorafenib 450 mg) are described below.
The major efficacy outcome measure was progression-free survival (PFS), as assessed by a blinded independent central review, to compare MEKTOVI in combination with encorafenib with vemurafenib. Additional efficacy measures included overall survival (OS), as well as objective response rate (ORR) and duration of response (DoR) which were assessed by central review.
A total of 577 patients were randomized, 192 to the MEKTOVI in combination with encorafenib arm, 194 to the encorafenib arm, and 191 to the vemurafenib arm. Of the 383 patients randomized to either the MEKTOVI in combination with encorafenib or the vemurafenib arms, the median age was 56 years (20 to 89 years), 59% were male, 91% were White, and 72% had baseline ECOG performance status of 0. Ninety-five percent (95%) had metastatic disease, 65% were Stage IVM1c, and 4% received prior CTLA-4, PD-1, or PD-L1 directed antibodies. Twenty-eight percent (28%) had elevated baseline serum lactate dehydrogenase (LDH), 45% had ≥3 organs with tumor involvement at baseline, and 3% had brain metastases. Based on centralized testing, 100% of patients’ tumors tested positive for BRAF mutations; BRAF V600E (88%), BRAF V600K (11%), or both (<1%).
MEKTOVI in combination with encorafenib demonstrated a statistically significant improvement in PFS compared to vemurafenib. Efficacy results are summarized in Table 7 and Figure 1.
| CI = Confidence interval; CR = Complete response; DoR = Duration of response; HR = Hazard ratio; NE = Not estimable; ORR = Overall response rate; OS = Overall survival; PFS = Progression-free survival; PR = Partial response. | ||
| ||
MEKTOVI with encorafenib N=192 | Vemurafenib N=191 | |
Progression-Free Survival | ||
Number of events (%) | 98 (51) | 106 (55) |
Progressive disease | 88 (46) | 104 (54) |
Death | 10 (5) | 2 (1) |
Median PFS, months (95% CI) | 14.9 (11, 18.5) | 7.3 (5.6, 8.2) |
HR (95% CI)* | 0.54 (0.41, 0.71) | |
P value† | <0.0001 | |
Overall Survival‡ | ||
Number of events (%) | 105 (55) | 127 (67) |
Median OS, months (95% CI) | 33.6 (22.4, 39.2) | 16.9 (14.0, 24.5) |
HR (95% CI) * | 0.61 (0.47, 0.79) | |
Overall Response Rate | ||
ORR (95% CI) | 63% (56%, 70%) | 40% (33%, 48%) |
CR | 8% | 6% |
PR | 55% | 35% |
Duration of Response | ||
Median DoR, months (95% CI) | 16.6 (12.2, 20.4) | 12.3 (6.9, 16.9) |
Figure 1: Kaplan-Meier Curves for Progression-Free Survival in COLUMBUS
14.2 BRAF V600E Mutation-Positive Metastatic Non-Small Cell Lung Cancer
MEKTOVI in combination with encorafenib was evaluated in an open-label, multicenter, single-arm study in patients with BRAF V600E mutation-positive metastatic non-small cell lung cancer (NSCLC) (PHAROS; NCT03915951). Eligible patients had a diagnosis of histologically-confirmed metastatic NSCLC with BRAF V600E mutation that was treatment-naïve or had been previously treated with 1 prior line of systemic therapy in the metastatic setting (platinum-based chemotherapy and/or anti-PD-1/PD-L1 therapies), age 18 years or older, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, and measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Prior use of BRAF inhibitors or MEK inhibitors was not allowed.
Patients received MEKTOVI 45 mg orally twice daily and encorafenib 450 mg once daily until disease progression or unacceptable toxicity. The major efficacy outcome measures were objective response rate (ORR) per RECIST v1.1 and duration of response (DoR) as assessed by independent review committee (IRC).
In the efficacy population, BRAF V600E mutation status was determined by prospective local testing using tumor tissue (78%) or blood (22%) specimens. Of the 98 patients with BRAF V600E mutation, 6 patients were enrolled into the trial based on testing of their tumor tissue specimens with the FoundationOne CDx tissue test. Of the remaining 92 patients enrolled based on local testing, 68 patients had their tumor tissue specimens retrospectively confirmed as having BRAF V600E positive status by the FoundationOne CDx tissue test. The remaining patients had either BRAF V600E negative status (n=5) or had unevaluable results (n=19) by the FoundationOne CDx tissue test. In addition, plasma samples from 81 out of 98 patients were retrospectively tested using the FoundationOne Liquid CDx assay. Of the 81 patients, 48 were confirmed positive for BRAF V600E, while 33 patients were BRAF V600E mutation negative by FoundationOne Liquid CDx assay. The remaining 17 samples had unevaluable results with FoundationOne Liquid CDx assay.
The efficacy population included 59 treatment-naïve patients and 39 previously-treated patients. Among these 98 patients, the median age was 70 years (range: 47 to 86); 53% female; 88% White, 7% Asian, 3% Black or African American, and 1% American Indian or Alaska Native; 99% were not Hispanic or Latino; 13% were current smokers and 57% were former smokers; 73% had ECOG PS of 1; and 97% had adenocarcinoma. All patients had metastatic disease and 8% had brain metastases at baseline.
Efficacy results for patients with BRAF V600E mutation-positive metastatic NSCLC are summarized in Table 8.
| CI = Confidence interval; CR = Complete response; DoR = Duration of response; N = Number of patients; NE = Not estimable; ORR = Objective response rate; PR = Partial response. | ||
| ||
MEKTOVI with encorafenib | ||
Efficacy Parameter | Treatment naïve (N=59) | Previously treated (N=39) |
Objective Response Rate* | ||
ORR (95% CI) | 75% (62, 85) | 46% (30, 63) |
CR | 15% | 10% |
PR | 59% | 36% |
Duration of Response* | N=44 | N=18 |
Median DoR, months | NE (23.1, NE) | 16.7 (7.4, NE) |
% with DoR ≥6 months | 75% | 67% |
% with DoR ≥12 months | 59% | 33% |
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.