LORBRENA® Clinical Studies
(lorlatinib)
14 CLINICAL STUDIES
Previously Untreated ALK-Positive Metastatic NSCLC (CROWN Study)
The efficacy of LORBRENA for the treatment of patients with ALK-positive NSCLC who had not received prior systemic therapy for metastatic disease was established in an open-label, randomized, active-controlled, multicenter study (Study B7461006; NCT03052608). Patients were required to have an ECOG performance status of 0–2 and ALK-positive NSCLC as identified by the VENTANA ALK (D5F3) CDx assay. Neurologically stable patients with treated or untreated asymptomatic CNS metastases, including leptomeningeal metastases, were eligible. Patients were required to have finished radiation therapy, at least 2 weeks (for stereotactic or partial radiation) or 4 weeks (for whole brain irradiation) prior to randomization. Patients with severe acute or chronic psychiatric conditions, including recent (within the past year) or active suicidal ideation or behavior, were excluded.
Patients were randomized 1:1 to receive LORBRENA 100 mg orally once daily or crizotinib 250 mg orally twice daily. Randomization was stratified by ethnic origin (Asian vs. non-Asian) and the presence or absence of CNS metastases at baseline. Treatment on both arms was continued until disease progression or unacceptable toxicity. The major efficacy outcome measure was progression-free survival (PFS) as determined by Blinded Independent Central Review (BICR) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (v1.1). Additional efficacy outcome measures were overall survival (OS) and tumor assessment related data by BICR, including overall response rate (ORR), and duration of response (DOR). In patients with measurable CNS metastases at baseline, additional outcome measures were intracranial overall response rate (IC-ORR) and intracranial duration of response (IC-DOR) by BICR.
A total of 296 patients were randomized to LORBRENA (n=149) or crizotinib (n=147). The demographic characteristics of the overall study population were: median age 59 years (range: 26 to 90 years), age ≥65 years (35%), 59% female, 49% White, 44% Asian, and 0.3% Black. The ECOG performance status at baseline was 0 or 1 in 96% of patients. The majority of patients had adenocarcinoma (95%) and never smoked (59%). CNS metastases were present in 26% (n=78) of patients: of these, 30 patients had measurable CNS lesions.
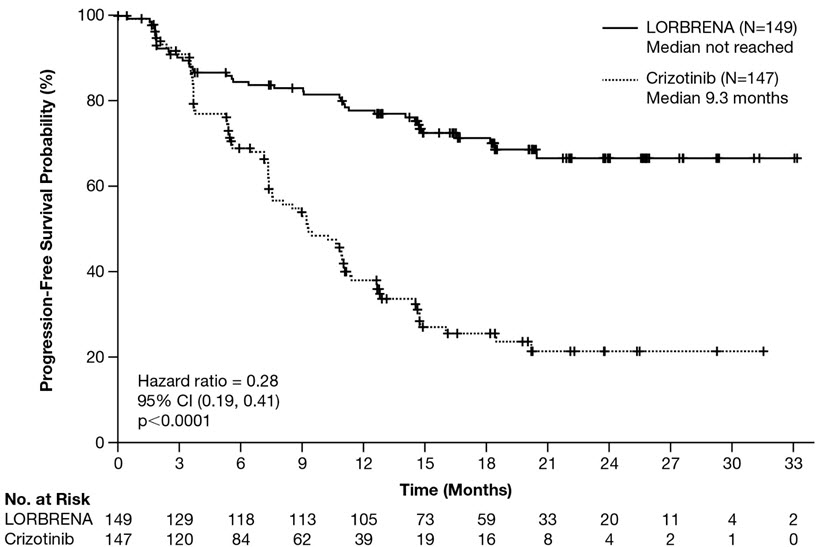
Efficacy results from Study B7461006 as assessed by BICR are summarized in Table 6 and Figure 1. Results demonstrated a significant improvement in PFS for the LORBRENA arm over the crizotinib arm. At the data cutoff point OS data was not mature.
| Efficacy Parameter | LORBRENA N=149 | Crizotinib N=147 |
|---|---|---|
| Abbreviations: CI=confidence interval; N=number of patients; NE=not estimable; PFS=progression free survival. | ||
Progression-free survival | ||
Number of events, n (%) | 41 (28%) | 86 (59%) |
Progressive disease, n (%) | 32 (22%) | 82 (56%) |
Death, n (%) | 9 (6%) | 4 (3%) |
Median, months (95% CI)* | NE (NE, NE) | 9.3 (7.6, 11.1) |
Hazard ratio (95% CI)† | 0.28 (0.19, 0.41) | |
p-value‡ | <0.0001 | |
Overall response rate | ||
Overall response rate (95% CI)§ | 76% (68, 83) | 58% (49, 66) |
Complete response | 3% | 0% |
Partial response | 73% | 58% |
Duration of response | ||
Number of responders, n | 113 | 85 |
Median, months (Range) | NE (0.9, 31.3) | 11 (1.1, 27.5) |
Response duration ≥6 months, n (%) | 101 (89%) | 53 (62%) |
Response duration ≥12 months, n (%) | 79 (70%) | 23 (27%) |
Response duration ≥18 months, n (%) | 34 (30%) | 9 (11%) |
Figure 1: Kaplan-Meier Plot of Progression-Free Survival by BICR in Study B7461006 (CROWN)
The results of prespecified exploratory analyses of intracranial response rate in 30 patients with measurable CNS lesions at baseline as assessed by BICR are summarized in Table 7.
| Intracranial Tumor Response Assessment | LORBRENA N=17 | Crizotinib N=13 |
|---|---|---|
| Abbreviations: CI=confidence interval; N/n=number of patients. | ||
| ||
Intracranial response rate (95% CI)* | 82% (57, 96) | 23% (5, 54) |
Complete response | 71% | 8% |
Duration of response | ||
Number of responders, n | 14 | 3 |
Response duration ≥12 months, n (%) | 11 (79%) | 0 |
ALK-Positive Metastatic NSCLC Previously Treated with an ALK Kinase Inhibitor
The efficacy of LORBRENA was demonstrated in a subgroup of patients with ALK-positive metastatic NSCLC previously treated with one or more ALK kinase inhibitors who were enrolled in a non-randomized, dose-ranging and activity-estimating, multi-cohort, multicenter study (Study B7461001; NCT01970865). Patients included in this subgroup were required to have metastatic disease with at least 1 measurable target lesion according to RECIST v1.1, ECOG performance status of 0 to 2, and documented ALK rearrangement in tumor tissue as determined by fluorescence in situ hybridization (FISH) assay or by Immunohistochemistry (IHC), and received LORBRENA 100 mg orally once daily. Patients with asymptomatic CNS metastases, including patients with stable or decreasing steroid use within 2 weeks prior to study entry, were eligible. Patients with severe, acute, or chronic psychiatric conditions including suicidal ideation or behavior were excluded. In addition, for patients with ALK-positive metastatic NSCLC, the extent and type of prior treatment was specified for each individual cohort (see Table 8). The major efficacy outcome measures were ORR and intracranial ORR, according to RECIST v1.1, as assessed by Independent Central Review (ICR) committee. Data were pooled across all subgroups listed in Table 8. Additional efficacy outcome measures included DOR, and intracranial DOR.
A total of 215 patients were enrolled across the subgroups in Table 8. The distribution of patients by type and extent of prior therapy is provided in Table 8. The demographic characteristics across all 215 patients were: 59% female, 51% White, 34% Asian, and the median age was 53 years (29 to 85 years) with 18% of patients ≥65 years. The ECOG performance status at baseline was 0 or 1 in 96% of patients. All patients had metastatic disease and 95% had adenocarcinoma. Brain metastases as identified by ICR were present in 69% of patients; of these, 60% had received prior radiation to the brain and 60% (n=89) had measurable disease per ICR.
| Extent of prior therapy | Number of patients |
|---|---|
| Abbreviations: ALK=anaplastic lymphoma kinase; NSCLC=non-small cell lung cancer. | |
| |
Prior crizotinib and no prior chemotherapy* | 29 |
Prior crizotinib and 1–2 lines of prior chemotherapy* | 35 |
Prior ALK inhibitor (not crizotinib) with or without prior chemotherapy* | 28 |
Two prior ALK inhibitors with or without prior chemotherapy* | 75 |
Three prior ALK inhibitors with or without prior chemotherapy* | 48 |
Total | 215 |
Efficacy results for Study B7461001 are summarized in Tables 9 and 10.
| Efficacy Parameter | Overall N=215 |
|---|---|
| Abbreviations: CI=confidence interval; N=number of patients. | |
48% (42, 55) | |
Complete response | 4% |
Partial response | 44% |
Duration of response | |
Median, months‡ (95% CI) | 12.5 (8.4, 23.7) |
An assessment of intracranial ORR and the duration of response for CNS metastases in the subgroup of 89 patients in Study B7461001 with baseline measurable lesions in the CNS according to RECIST v1.1 are summarized in Table 10. Of these, 56 (63%) patients received prior brain radiation, including 42 patients (47%) who completed brain radiation treatment at least 6 months before starting treatment with LORBRENA.
| Efficacy Parameter | Intracranial N=89 |
|---|---|
| Abbreviations: CI=confidence interval; N=number of patients; NR=not reached. | |
60% (49, 70) | |
Complete response | 21% |
Partial response | 38% |
Duration of response | |
Median, months‡ (95% CI) | 19.5 (12.4, NR) |
In exploratory analyses conducted in subgroups defined by prior therapy, the response rates to LORBRENA were:
- •
- ORR = 39% (95% CI: 30, 48) in 119 patients who received crizotinib and at least one other ALK inhibitor, with or without prior chemotherapy
- •
- ORR = 31% (95% CI: 9, 61) in 13 patients who received alectinib as their only ALK inhibitor, with or without prior chemotherapy
- •
- ORR = 46% (95% CI: 19, 75) in 13 patients who received ceritinib as their only ALK inhibitor, with or without prior chemotherapy
Find LORBRENA® medical information:
Find LORBRENA® medical information:
LORBRENA® Quick Finder
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
Previously Untreated ALK-Positive Metastatic NSCLC (CROWN Study)
The efficacy of LORBRENA for the treatment of patients with ALK-positive NSCLC who had not received prior systemic therapy for metastatic disease was established in an open-label, randomized, active-controlled, multicenter study (Study B7461006; NCT03052608). Patients were required to have an ECOG performance status of 0–2 and ALK-positive NSCLC as identified by the VENTANA ALK (D5F3) CDx assay. Neurologically stable patients with treated or untreated asymptomatic CNS metastases, including leptomeningeal metastases, were eligible. Patients were required to have finished radiation therapy, at least 2 weeks (for stereotactic or partial radiation) or 4 weeks (for whole brain irradiation) prior to randomization. Patients with severe acute or chronic psychiatric conditions, including recent (within the past year) or active suicidal ideation or behavior, were excluded.
Patients were randomized 1:1 to receive LORBRENA 100 mg orally once daily or crizotinib 250 mg orally twice daily. Randomization was stratified by ethnic origin (Asian vs. non-Asian) and the presence or absence of CNS metastases at baseline. Treatment on both arms was continued until disease progression or unacceptable toxicity. The major efficacy outcome measure was progression-free survival (PFS) as determined by Blinded Independent Central Review (BICR) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (v1.1). Additional efficacy outcome measures were overall survival (OS) and tumor assessment related data by BICR, including overall response rate (ORR), and duration of response (DOR). In patients with measurable CNS metastases at baseline, additional outcome measures were intracranial overall response rate (IC-ORR) and intracranial duration of response (IC-DOR) by BICR.
A total of 296 patients were randomized to LORBRENA (n=149) or crizotinib (n=147). The demographic characteristics of the overall study population were: median age 59 years (range: 26 to 90 years), age ≥65 years (35%), 59% female, 49% White, 44% Asian, and 0.3% Black. The ECOG performance status at baseline was 0 or 1 in 96% of patients. The majority of patients had adenocarcinoma (95%) and never smoked (59%). CNS metastases were present in 26% (n=78) of patients: of these, 30 patients had measurable CNS lesions.
Efficacy results from Study B7461006 as assessed by BICR are summarized in Table 6 and Figure 1. Results demonstrated a significant improvement in PFS for the LORBRENA arm over the crizotinib arm. At the data cutoff point OS data was not mature.
| Efficacy Parameter | LORBRENA N=149 | Crizotinib N=147 |
|---|---|---|
| Abbreviations: CI=confidence interval; N=number of patients; NE=not estimable; PFS=progression free survival. | ||
Progression-free survival | ||
Number of events, n (%) | 41 (28%) | 86 (59%) |
Progressive disease, n (%) | 32 (22%) | 82 (56%) |
Death, n (%) | 9 (6%) | 4 (3%) |
Median, months (95% CI)* | NE (NE, NE) | 9.3 (7.6, 11.1) |
Hazard ratio (95% CI)† | 0.28 (0.19, 0.41) | |
p-value‡ | <0.0001 | |
Overall response rate | ||
Overall response rate (95% CI)§ | 76% (68, 83) | 58% (49, 66) |
Complete response | 3% | 0% |
Partial response | 73% | 58% |
Duration of response | ||
Number of responders, n | 113 | 85 |
Median, months (Range) | NE (0.9, 31.3) | 11 (1.1, 27.5) |
Response duration ≥6 months, n (%) | 101 (89%) | 53 (62%) |
Response duration ≥12 months, n (%) | 79 (70%) | 23 (27%) |
Response duration ≥18 months, n (%) | 34 (30%) | 9 (11%) |
Figure 1: Kaplan-Meier Plot of Progression-Free Survival by BICR in Study B7461006 (CROWN)
The results of prespecified exploratory analyses of intracranial response rate in 30 patients with measurable CNS lesions at baseline as assessed by BICR are summarized in Table 7.
| Intracranial Tumor Response Assessment | LORBRENA N=17 | Crizotinib N=13 |
|---|---|---|
| Abbreviations: CI=confidence interval; N/n=number of patients. | ||
| ||
Intracranial response rate (95% CI)* | 82% (57, 96) | 23% (5, 54) |
Complete response | 71% | 8% |
Duration of response | ||
Number of responders, n | 14 | 3 |
Response duration ≥12 months, n (%) | 11 (79%) | 0 |
ALK-Positive Metastatic NSCLC Previously Treated with an ALK Kinase Inhibitor
The efficacy of LORBRENA was demonstrated in a subgroup of patients with ALK-positive metastatic NSCLC previously treated with one or more ALK kinase inhibitors who were enrolled in a non-randomized, dose-ranging and activity-estimating, multi-cohort, multicenter study (Study B7461001; NCT01970865). Patients included in this subgroup were required to have metastatic disease with at least 1 measurable target lesion according to RECIST v1.1, ECOG performance status of 0 to 2, and documented ALK rearrangement in tumor tissue as determined by fluorescence in situ hybridization (FISH) assay or by Immunohistochemistry (IHC), and received LORBRENA 100 mg orally once daily. Patients with asymptomatic CNS metastases, including patients with stable or decreasing steroid use within 2 weeks prior to study entry, were eligible. Patients with severe, acute, or chronic psychiatric conditions including suicidal ideation or behavior were excluded. In addition, for patients with ALK-positive metastatic NSCLC, the extent and type of prior treatment was specified for each individual cohort (see Table 8). The major efficacy outcome measures were ORR and intracranial ORR, according to RECIST v1.1, as assessed by Independent Central Review (ICR) committee. Data were pooled across all subgroups listed in Table 8. Additional efficacy outcome measures included DOR, and intracranial DOR.
A total of 215 patients were enrolled across the subgroups in Table 8. The distribution of patients by type and extent of prior therapy is provided in Table 8. The demographic characteristics across all 215 patients were: 59% female, 51% White, 34% Asian, and the median age was 53 years (29 to 85 years) with 18% of patients ≥65 years. The ECOG performance status at baseline was 0 or 1 in 96% of patients. All patients had metastatic disease and 95% had adenocarcinoma. Brain metastases as identified by ICR were present in 69% of patients; of these, 60% had received prior radiation to the brain and 60% (n=89) had measurable disease per ICR.
| Extent of prior therapy | Number of patients |
|---|---|
| Abbreviations: ALK=anaplastic lymphoma kinase; NSCLC=non-small cell lung cancer. | |
| |
Prior crizotinib and no prior chemotherapy* | 29 |
Prior crizotinib and 1–2 lines of prior chemotherapy* | 35 |
Prior ALK inhibitor (not crizotinib) with or without prior chemotherapy* | 28 |
Two prior ALK inhibitors with or without prior chemotherapy* | 75 |
Three prior ALK inhibitors with or without prior chemotherapy* | 48 |
Total | 215 |
Efficacy results for Study B7461001 are summarized in Tables 9 and 10.
| Efficacy Parameter | Overall N=215 |
|---|---|
| Abbreviations: CI=confidence interval; N=number of patients. | |
48% (42, 55) | |
Complete response | 4% |
Partial response | 44% |
Duration of response | |
Median, months‡ (95% CI) | 12.5 (8.4, 23.7) |
An assessment of intracranial ORR and the duration of response for CNS metastases in the subgroup of 89 patients in Study B7461001 with baseline measurable lesions in the CNS according to RECIST v1.1 are summarized in Table 10. Of these, 56 (63%) patients received prior brain radiation, including 42 patients (47%) who completed brain radiation treatment at least 6 months before starting treatment with LORBRENA.
| Efficacy Parameter | Intracranial N=89 |
|---|---|
| Abbreviations: CI=confidence interval; N=number of patients; NR=not reached. | |
60% (49, 70) | |
Complete response | 21% |
Partial response | 38% |
Duration of response | |
Median, months‡ (95% CI) | 19.5 (12.4, NR) |
In exploratory analyses conducted in subgroups defined by prior therapy, the response rates to LORBRENA were:
- •
- ORR = 39% (95% CI: 30, 48) in 119 patients who received crizotinib and at least one other ALK inhibitor, with or without prior chemotherapy
- •
- ORR = 31% (95% CI: 9, 61) in 13 patients who received alectinib as their only ALK inhibitor, with or without prior chemotherapy
- •
- ORR = 46% (95% CI: 19, 75) in 13 patients who received ceritinib as their only ALK inhibitor, with or without prior chemotherapy
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.