11 DESCRIPTION
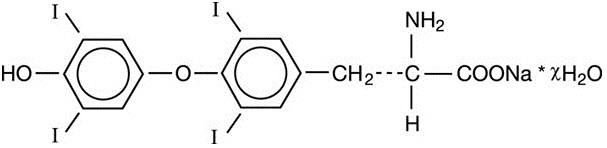
LEVOXYL contains the active ingredient, levothyroxine, asynthetic crystalline levothyroxine (T4) in sodium salt form. It is chemically designated as L-3,3',5,5'-tetraiodothyronine monosodium hydrate. Synthetic T4 is identical in chemical structure to the T4 produced in the human thyroid gland. Levothyroxine sodium has an empirical formula of C15H10I4N NaO4 ∙ H2O, molecular weight of 798.85 g/mol (anhydrous), and structural formula as shown:
LEVOXYL tablets for oral administration are supplied in the following strengths: 25 mcg, 50 mcg, 75 mcg, 88 mcg, 100 mcg, 112 mcg, 125 mcg, 137 mcg, 150 mcg, 175 mcg, and 200 mcg.
Inactive Ingredients
Calcium sulfate dihydrate, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and sodium bicarbonate. The following are the coloring additives per tablet strength:
Strength
(mcg) | Color additive(s) |
|---|
| 25 | FD&C Yellow No. 6 Aluminum Lake |
| 50 | None |
| 75 | FD&C Blue No. 1 Aluminum Lake, D&C Red No. 30 Aluminum Lake |
| 88 | FD&C Yellow No. 6 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake, D&C Yellow No. 10 Aluminum Lake |
| 100 | FD&C Yellow No. 6 Aluminum Lake, D&C Yellow No. 10 Aluminum Lake |
| 112 | FD&C Yellow No. 6 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, D&C Red No. 30 Aluminum Lake |
| 125 | FD&C Red No. 40 Aluminum Lake, D&C Yellow No. 10 Aluminum Lake |
| 137 | FD&C Blue No. 1 Aluminum Lake |
| 150 | FD&C Blue No. 1 Aluminum Lake, D&C Red No. 30 Aluminum Lake |
| 175 | FD&C Blue No. 1 Aluminum Lake, D&C Yellow No. 10 Aluminum Lake |
| 200 | D&C Red No. 30 Aluminum Lake, D&C Yellow No. 10 Aluminum Lake |