ketamine hydrochloride injection, USP
()
Find ketamine hydrochloride injection, USP medical information:
Find ketamine hydrochloride injection, USP medical information:
ketamine hydrochloride injection, USP Quick Finder
Highlights
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KETAMINE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for KETAMINE HYDROCHLORIDE INJECTION. KETAMINE HYDROCHLORIDE injection, for intravenous or intramuscular use, CIII Initial U.S. Approval: 1970 RECENT MAJOR CHANGES
INDICATIONS AND USAGEKetamine Hydrochloride Injection is a general anesthetic indicated: DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSInjection: 500 mg/10 mL (50 mg/mL) and 500 mg/5 mL (100 mg/mL) multiple-dose vials. (3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions are emergence reactions and elevated blood pressure and pulse. (6) To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION. Revised: 7/2022 |
Indications and Usage
1 INDICATIONS AND USAGE
Ketamine Hydrochloride Injection is indicated:
- •
- as the sole anesthetic agent for diagnostic and surgical procedures that do not require skeletal muscle relaxation.
- •
- for the induction of anesthesia prior to the administration of other general anesthetic agents.
- •
- as a supplement to other anesthetic agents.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
Ketamine Hydrochloride Injection should be administered by or under the direction of physicians experienced in the administration of general anesthetics, maintenance of a patent airway, and oxygenation and ventilation. Continuously monitor vital signs in patients receiving Ketamine Hydrochloride Injection.
Emergency airway equipment must be immediately available.
Do not administer the 100 mg/mL concentration of Ketamine Hydrochloride Injection intravenously without proper dilution [see Dosage and Administration (2.3)]. Must be used immediately after dilution.
While some degree of airway protection may be afforded due to active laryngeal-pharyngeal reflexes, vomiting and aspiration may occur with ketamine. Ketamine Hydrochloride Injection is not recommended for use in patients who have not followed nil per os guidelines.
Due to the potential for salivation during Ketamine Hydrochloride Injection administration, administer an antisialagogue prior to induction of anesthesia.
In individuals with a history of chronic ketamine use for off-label indications, there have been case reports of genitourinary pain that may be related to the ketamine treatment, not the underlying condition [see Adverse Reactions (6)]. Consider cessation of ketamine if genitourinary pain continues in the setting of other genitourinary symptoms.
2.2 Recommended Dosage and Administration
The Ketamine Hydrochloride Injection dosage must be individualized and titrated to the desired clinical effect.
If a longer duration of effect is desired, additional increments can be administered intravenously or intramuscularly to maintain anesthesia. However, a higher total dose will result in a longer time to complete recovery.
Induction of Anesthesia
Intravenous Route: The initial dose of Ketamine Hydrochloride Injection administered intravenously may range from 1 mg/kg to 4.5 mg/kg. The average amount required to produce 5 to 10 minutes of surgical anesthesia within 30 seconds following injection is 2 mg/kg. Administer Ketamine Hydrochloride Injection slowly (i.e., over a period of 60 seconds). Rapid administration may result in respiratory depression and enhanced vasopressor response. The induction dose may be administered as an intravenous infusion at a rate of 0.5 mg/kg/min.
Intramuscular Route: The initial dose of Ketamine Hydrochloride Injection administered intramuscularly may range from 6.5 to 13 mg/kg. A dose of 9 to 13 mg/kg usually produces surgical anesthesia within 3 to 4 minutes following injection, with the anesthetic effect usually lasting 12 to 25 minutes. Administer a benzodiazepine, if clinically indicated, for the prevention of neuropsychological manifestations during emergence from anesthesia.
Maintenance of Anesthesia
Adjust the maintenance dose according to the patient's anesthetic needs and whether an additional anesthetic agent is administered.
Repeat increments of one-half to the full induction dose as needed for maintenance of anesthesia. Purposeless and tonic-clonic movements of extremities may occur during the course of ketamine anesthesia. These movements do not imply a light plane and are not indicative of the need for additional doses of the anesthetic.
Ketamine Hydrochloride Injection given by slow microdrip infusion technique at a dose of 0.1 to 0.5 mg/minute will maintain general anesthesia in adult patients induced with ketamine. Augment Ketamine Hydrochloride Injection with an intravenous benzodiazepine for the prevention of neuropsychological manifestations during emergence.
Supplement to Other Anesthetic Agents
Ketamine Hydrochloride Injection can be administered to supplement other general and local anesthetic agents. Continuously monitor patients for changes in respiratory and hemodynamic parameters.
A reduced dose of Ketamine Hydrochloride Injection can be used to produce balanced anesthesia when used in combination with other anesthetic agents.
2.3 Preparation of Dilution
Ketamine Hydrochloride Injection is a clear, colorless sterile solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard if product is discolored or contains particulate matter.
Induction of Anesthesia: Do not intravenously inject the 100 mg/mL concentration of Ketamine Hydrochloride Injection without proper dilution. Dilute Ketamine Hydrochloride Injection with an equal volume of either Sterile Water for injection, USP, 0.9% Sodium Chloride Injection, USP (Normal Saline), or 5% Dextrose in Water. Use immediately after dilution.
Maintenance of Anesthesia: To prepare a dilute solution containing 1 mg of ketamine per mL, aseptically transfer 10 mL from a 50 mg per mL vial or 5 mL from a 100 mg per mL vial to 500 mL of 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP (Normal Saline) and mix well. The resultant solution will contain 1 mg of ketamine per mL. Use immediately after dilution.
When fluid restriction is required, Ketamine Hydrochloride Injection can be added to a 250 mL infusion as described above to provide a Ketamine Hydrochloride Injection concentration of 2 mg/mL.
Dosage Forms and Strengths
3 DOSAGE FORMS AND STRENGTHS
Ketamine Hydrochloride Injection, USP is a clear, colorless, sterile solution available in multiple-dose vials containing either 50 mg ketamine base/mL (equivalent to 57.7 mg ketamine hydrochloride) or 100 mg ketamine base/mL (equivalent to 115.3 mg ketamine hydrochloride).
- •
- 500 mg/10 mL (50 mg/mL)
- •
- 500 mg/5 mL (100 mg/mL)
Contraindications
4 CONTRAINDICATIONS
- •
- Ketamine Hydrochloride Injection is contraindicated in patients for whom a significant elevation of blood pressure would constitute a serious hazard [see Warnings and Precautions (5.1)].
- •
- Ketamine Hydrochloride Injection is contraindicated in patients with known hypersensitivity to ketamine or to any excipient [see Adverse Reactions (6)].
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Hemodynamic Instability
Transient increases in blood pressure, heart rate, and cardiac index are frequently observed following administration of Ketamine Hydrochloride Injection. Decreases in blood pressure and heart rate, arrhythmias, and cardiac decompensation have also been observed. Monitor vital signs and cardiac function during Ketamine Hydrochloride Injection administration. Ketamine Hydrochloride Injection is contraindicated in patients for whom a significant elevation of blood pressure would constitute a serious hazard [see Contraindications (4)].
5.2 Emergence Reactions
Emergence delirium (postoperative confusional states or agitation) has occurred in approximately 12% of patients during the recovery period, and the duration is generally a few hours. The neuropsychological manifestations vary in severity between pleasant dream-like states, vivid imagery, hallucinations, and emergence delirium. In some cases, these states have been accompanied by confusion, excitement, and irrational behavior, which have been recalled as unpleasant experiences. No residual psychological effects are known to have resulted from use of Ketamine Hydrochloride Injection during induction and maintenance of anesthesia.
Intramuscular administration results in a lower incidence of emergence reactions.
The incidence of psychological manifestations during emergence, particularly dream-like observations and emergence delirium, may be reduced by using lower recommended dosages of Ketamine Hydrochloride Injection in conjunction with an intravenous benzodiazepine during induction and maintenance of anesthesia [see Dosage and Administration (2.3)]. Also, these reactions may be reduced if verbal, tactile, and visual stimulation of the patient is minimized during the recovery period. This does not preclude the monitoring of vital signs.
5.3 Respiratory Depression
Respiratory depression may occur with overdosage or a rapid rate of administration of Ketamine Hydrochloride Injection. Maintain adequate oxygenation and ventilation.
5.4 Risks of Ketamine Hydrochloride Injection Alone for Procedures of the Pharynx, Larynx, or Bronchial Tree
Ketamine Hydrochloride Injection does not suppress pharyngeal and laryngeal reflexes. Avoid Ketamine Hydrochloride Injection administration as a sole anesthetic agent during procedures of the pharynx, larynx, or bronchial tree, including mechanical stimulation of the pharynx. Muscle relaxants may be required for successful completion of procedures of the pharynx, larynx, or bronchial tree.
5.5 Pediatric Neurotoxicity
Published animal studies demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity increase neuronal apoptosis in the developing brain and result in long-term cognitive deficits when used for longer than 3 hours. The clinical significance of these findings is not clear. However, based on the available data, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately three years of age in humans [see Use in Specific Populations (8.1, 8.4), Nonclinical Toxicology (13.2)].
Some published studies in children suggest that similar deficits may occur after repeated or prolonged exposures to anesthetic agents early in life and may result in adverse cognitive or behavioral effects. These studies have substantial limitations, and it is not clear if the observed effects are due to the anesthetic/sedation drug administration or other factors such as the surgery or underlying illness.
Anesthetic and sedation drugs are a necessary part of the care of children needing surgery, other procedures, or tests that cannot be delayed, and no specific medications have been shown to be safer than any other. Decisions regarding the timing of any elective procedures requiring anesthesia should take into consideration the benefits of the procedure weighed against the potential risks.
5.6 Drug-Induced Liver Injury
Ketamine administration is associated with hepatobiliary dysfunction (most often a cholestatic pattern), with recurrent use (e.g., misuse/abuse or medically supervised unapproved indications). Biliary duct dilatation with or without evidence of biliary obstruction has also been reported with recurrent use. Obtain baseline LFTs, including alkaline phosphatase and gamma glutamyl transferase, in patients receiving ketamine as part of a treatment plan that utilizes recurrent dosing. Monitor those receiving recurrent ketamine at periodic intervals during treatment.
5.7 Increase in Cerebrospinal Fluid Pressure
An increase in intracranial pressure has been reported following administration of Ketamine Hydrochloride Injection. Patients with elevated intracranial pressure should be in a monitored setting with frequent neurologic assessments.
5.8 Drug Interactions
Theophylline or Aminophylline: Concomitant administration of Ketamine Hydrochloride Injection and theophylline or aminophylline may lower the seizure threshold [see Drug Interactions (7.1)]. Consider using an alternative to Ketamine Hydrochloride Injection in patients receiving theophylline or aminophylline.
Sympathomimetics and Vasopressin: Sympathomimetics and vasopressin may enhance the sympathomimetic effects of ketamine [see Drug Interactions (7.2)]. Closely monitor vital signs when Ketamine Hydrochloride Injection and sympathomimetics or vasopressin are co-administered and consider dose adjustment individualized to the patient's clinical situation.
Benzodiazepines, Opioid Analgesics, or Other CNS Depressants
Concomitant use of ketamine with opioid analgesics, benzodiazepines, or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death [see Drug Interactions (7.3)]. Closely monitor neurological status and respiratory parameters, including respiratory rate and pulse oximetry, when Ketamine Hydrochloride Injection and opioid analgesics, benzodiazepines, or other CNS depressants are co-administered. Consider dose adjustment individualized to the patient's clinical situation.
Adverse Reactions
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Hemodynamic Instability [see Warnings and Precautions (5.1)]
- •
- Emergence Reactions [see Warnings and Precautions (5.2)]
- •
- Respiratory Depression [see Warnings and Precautions (5.3)]
- •
- Pediatric Neurotoxicity [see Warnings and Precautions (5.5)]
- •
- Drug-Induced Liver Injury [see Warnings and Precautions (5.6)]
The following adverse reactions associated with the use of Ketamine Hydrochloride Injection were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular disorders: Elevated blood pressure, heart rate, and cardiac index; decreases in blood pressure and heart rate; arrhythmias; cardiac decompensation (in patients with suspected catecholamine depletion).
Eye disorders: Diplopia, nystagmus, elevation in intraocular pressure.
Gastrointestinal disorders: Anorexia, nausea, vomiting, hepatobiliary dysfunction. Biliary duct dilatation with or without evidence of biliary obstruction has been reported with recurrent use (e.g., misuse/abuse or medically supervised unapproved indications).
Administration site disorders: Local pain and exanthema at the injection site.
Immune system disorders: Anaphylaxis.
Neurologic disorders: Emergence reactions (post-operative delirium), [see Warnings and Precautions (5.2)]. During administration, enhanced muscle tone and spasms (resembling a partial motor or generalized motor seizure).
Psychiatric disorders: Adverse psychiatric events have occurred and/or persisted days to weeks after ketamine exposure.
Renal and urinary disorders: In individuals with history of chronic ketamine use or abuse, lower urinary tract and bladder symptoms including dysuria, increased urinary frequency, urgency, urge incontinence, and hematuria have been reported [see Dosage and Administration (2.1)]. In addition, diagnostic studies performed to assess the cause of these symptoms have reported cystitis (including cystitis non-infective, cystitis interstitial, cystitis ulcerative, cystitis erosive and cystitis hemorrhagic) as well as hydronephrosis and reduced bladder capacity.
Respiratory disorders: Respiratory depression and apnea following rapid intravenous administration of high doses of ketamine hydrochloride injection; laryngospasm, and airway obstruction.
Skin and subcutaneous tissue disorders: Transient erythema and/or morbilliform rash.
Drug Interactions
7 DRUG INTERACTIONS
7.1 Theophylline or Aminophylline
Concomitant administration of Ketamine Hydrochloride Injection and theophylline or aminophylline may lower the seizure threshold. Consider using an alternative to Ketamine Hydrochloride Injection in patients receiving theophylline or aminophylline.
7.2 Sympathomimetics and Vasopressin
Sympathomimetics and vasopressin may enhance the sympathomimetic effects of ketamine. Closely monitor vital signs when Ketamine Hydrochloride Injection and sympathomimetics or vasopressin are co-administered and consider dose adjustment individualized to the patient's clinical situation.
7.3 Benzodiazepines, Opioid Analgesics, Or Other CNS Depressants
Concomitant use of ketamine with opioid analgesics, benzodiazepines, or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death [see Warnings and Precautions (5.8)].
Opioid analgesics administered concomitantly with Ketamine Hydrochloride Injection may prolong time to complete recovery from anesthesia.
Use in Specific Populations
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of Ketamine Hydrochloride Injection in pregnant women. In animal reproduction studies in rats developmental delays (hypoplasia of skeletal tissues) were noted at 0.3 times the human intramuscular dose of 10 mg/kg. In rabbits, developmental delays and increased fetal resorptions were noted at 0.6 times the human dose. Published studies in pregnant primates demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity during the period of peak brain development increases neuronal apoptosis in the developing brain of the offspring when used for longer than 3 hours. There are no data on pregnancy exposures in primates corresponding to periods prior to the third trimester in humans.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Ketamine Hydrochloride Injection use in pregnancy, including obstetrics (either vaginal or abdominal delivery), is not recommended because safe use has not been established [see Warnings and Precautions (5.5), Use in Specific Populations (8.4), and Nonclinical Toxicology (13.2)].
Data
Animal Data
Pregnant rats were treated intramuscularly with 20 mg/kg ketamine (0.3 times the human dose of 10 mg/kg IM based on body surface area) on either Gestation Days 6 to 10 or Gestation Days 11 to 15. Ketamine treatment produced an increased incidence of hypoplastic skull, phalanges, and sternebrae in the pups.
Pregnant rabbits were treated intramuscularly with 20 mg/kg ketamine (0.6 times the human dose of 10 mg/kg IM based on body surface area) on either Gestation Days 6 to 10 or Gestation Days 11 to 15. An increase in resorptions and skeletal hypoplasia of the fetuses were noted. Additional pregnant rabbits were treated intramuscularly with a single dose 60 mg/kg (1.9 times the human dose of 10 mg/kg IM based on body surface area) on Gestation Day 6 only. Skeletal hypoplasia was reported in the fetuses.
In a study where pregnant rats were treated intramuscularly with 20 mg/kg ketamine (0.3 times the human dose of 10 mg/kg IM based on body surface area) from Gestation Day 18 to 21. There was a slight increase in incidence of delayed parturition by one day in treated dams of this group. No adverse effects on the litters or pups were noted; however, learning and memory assessments were not completed.
Three pregnant beagle dogs were treated intramuscularly with 25 mg/kg ketamine (1.3 times the human dose of 10 mg/kg IM based on body surface area) twice weekly for the three weeks of the first, second, and third trimesters of pregnancy, respectively, without the development of adverse effects in the pups.
In a published study in primates, administration of an anesthetic dose of ketamine for 24 hours on Gestation Day 122 increased neuronal apoptosis in the developing brain of the fetus. In other published studies, administration of either isoflurane or propofol for 5 hours on Gestation Day 120 resulted in increased neuronal and oligodendrocyte apoptosis in the developing brain of the offspring. With respect to brain development, this time period corresponds to the third trimester of gestation in the human. The clinical significance of these findings is not clear; however, studies in juvenile animals suggest neuroapoptosis correlates with long-term cognitive deficits [see Warnings and Precautions (5.5), Use in Specific Populations (8.4), and Nonclinical Toxicology (13.2)].
8.4 Pediatric Use
Safety and effectiveness in pediatric patients below the age of 16 have not been established.
Published juvenile animal studies demonstrate that the administration of anesthetic and sedation drugs, such as Ketamine Hydrochloride Injection, that either block NMDA receptors or potentiate the activity of GABA during the period of rapid brain growth or synaptogenesis, results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of ketamine that produced a light surgical plane of anesthesia did not increase neuronal cell loss, however, treatment regimens of 5 hours or longer of isoflurane increased neuronal cell loss. Data from isoflurane-treated rodents and ketamine-treated primates suggest that the neuronal and oligodendrocyte cell losses are associated with prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in neonates and young children who require procedures with the potential risks suggested by the nonclinical data [see Warnings and Precautions (5.5), Use in Specific Populations (8.1), and Nonclinical Toxicology (13.2)].
8.5 Geriatric Use
Clinical studies of ketamine hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Drug Abuse and Dependence
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Ketamine Hydrochloride Injection contains ketamine, a Schedule III controlled substance under the Controlled Substance Act.
9.2 Abuse
Individuals with a history of drug abuse or dependence may be at greater risk for abuse and misuse of Ketamine Hydrochloride Injection. Abuse is the intentional, non-therapeutic use of a drug, even once, for its psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed.
In a context of drug abuse, Ketamine Hydrochloride Injection may produce a variety of symptoms including anxiety, dysphoria, disorientation, insomnia, flashback, hallucinations, and feelings of floating, detachment and being "spaced out".
Recurrent high-dose ketamine misuse or abuse may be associated with memory and/or attention impairment.
9.3 Dependence
Physical dependence has been reported with prolonged use of ketamine. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or significant dosage reduction of a drug. Withdrawal symptoms have been reported after the discontinuation of frequently used (more than weekly), large doses of ketamine for long periods of time. Reported symptoms of withdrawal associated with daily intake of large doses of ketamine include craving, fatigue, poor appetite, and anxiety.
Tolerance has been reported with prolonged use of ketamine. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose).
Overdosage
10 OVERDOSAGE
Changes in heart rate and blood pressure, respiratory depression, and apnea may occur with overdosage or by a rapid rate of administration of Ketamine Hydrochloride Injection. Monitor patients for clinically relevant changes in heart rate and blood pressure. Assisted ventilation, including mechanical ventilation, may be required.
In cases of unintentional overdose of Ketamine Hydrochloride Injection (up to ten times that usually required), patients had a prolonged but complete recovery.
Description
11 DESCRIPTION
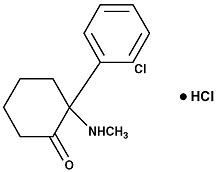
Ketamine Hydrochloride Injection, USP for intravenous or intramuscular use, contains ketamine, a nonbarbiturate general anesthetic. Ketamine hydrochloride, USP is a white crystalline powder and has a molecular formula of C13H16ClNO∙HCl and a molecular weight of 274.19. The chemical name for ketamine hydrochloride is (±)-2-(o-Chlorophenyl)-2-(methylamino)cyclohexanone hydrochloride. The chemical structure of ketamine hydrochloride is:
It is formulated as a slightly acidic (pH 3.5 to 5.5) sterile solution for intravenous or intramuscular injection. Each milliliter (mL) of the multiple-dose vials contain either 50 mg ketamine base (equivalent to 57.7 mg ketamine hydrochloride) or 100 mg ketamine base (equivalent to 115.3 mg ketamine hydrochloride) and not more than 0.10 mg/mL benzethonium chloride added as a preservative in water for injection.
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ketamine Hydrochloride Injection, a racemic mixture of ketamine, is a non-selective, non-competitive antagonist of the N-methyl-D-aspartate (NMDA) receptor, an ionotropic glutamate receptor. The major circulating metabolite of ketamine (norketamine) demonstrated activity at the same receptor with less affinity. Norketamine is about 1/3 as active as ketamine in reducing halothane requirements (MAC) of the rat.
12.2 Pharmacodynamics
Nervous System
Ketamine is a rapidly-acting general anesthetic producing a dissociative anesthetic state characterized by profound analgesia, normal pharyngeal-laryngeal reflexes, normal or slightly enhanced skeletal muscle tone, cardiovascular and respiratory stimulation, and occasionally a transient and minimal respiratory depression. The mechanism of action is primarily due to antagonism of N-methyl-D-aspartate (NMDA receptors) in the central nervous system.
Ketamine can produce nystagmus with pupillary dilation, salivation, lacrimation, and spontaneous limb movements with increased muscle tone through indirect sympathomimetic activity. Ketamine produces analgesia. Ketamine-induced emergence delirium can be reduced with benzodiazepines.
Cardiovascular System
Ketamine increases blood pressure, heart rate, and cardiac output. Cardiovascular effects of ketamine are indirect and believed to be mediated by inhibition of both central and peripheral catecholamine reuptake. Elevation of blood pressure reaches a maximum within a few minutes of injection and usually returns to preanesthetic values within 15 minutes. In the majority of cases, the systolic and diastolic blood pressure peaks from 10% to 50% above preanesthetic levels shortly after induction of anesthesia, but the elevation can be higher or longer in individual cases.
12.3 Pharmacokinetics
Distribution
Following intravenous administration, the ketamine concentration has an initial slope (alpha phase) lasting about 45 minutes with a half-life of 10 to 15 minutes. This first phase corresponds clinically to the anesthetic effect of the drug.
Elimination
Metabolism
Ketamine is metabolized via N-dealkylation to the active metabolite norketamine primarily by CYP2B6 and CYP3A4 and to a lesser extent by other CYP enzymes. Norketamine undergoes hydroxylation of the cyclohexone ring to form hydroxynorketamine compounds via CYP-dependent pathways, which are conjugated with glucuronic acid and subsequently undergo dehydration of the hydroxylated metabolites to form the cyclohexene derivative dehydroxynorketamine.
Excretion
Following intravenous administration, the ketamine concentration decreases due to a combination of redistribution from the CNS to slower equilibrating peripheral tissues and hepatic biotransformation to norketamine. The redistribution half-life of ketamine from the CNS to slower equilibrating peripheral tissues (beta phase) is 2.5 hours.
Nonclinical Toxicology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies have not been conducted to evaluate the carcinogenic potential of ketamine.
Mutagenesis
In a published report, ketamine was clastogenic in the in vitro chromosomal aberration assay.
Impairment of Fertility
Adequate studies to evaluate the impact of ketamine on male or female fertility have not been conducted. Male and female rats were treated with 10 mg/kg ketamine IV (0.8 times the average human induction dose of 2 mg/kg IV based on body surface area) on Days 11, 10, and 9 prior to mating. No impact on fertility was noted; however, this study design does not adequately characterize the impact of a drug on fertility endpoints.
13.2 Animal Toxicology and/or Pharmacology
Published studies in animals demonstrate that the use of anesthetic agents during the period of rapid brain growth or synaptogenesis results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester through the first several months of life, but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of an anesthetic regimen that produced a light surgical plane of anesthesia did not increase neuronal cell loss, however, treatment regimens of 5 hours or longer increased neuronal cell loss. Data in rodents and in primates suggest that the neuronal and oligodendrocyte cell losses are associated with subtle but prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in neonates and young children who require procedures against the potential risks suggested by the nonclinical data [see Warnings and Precautions (5.5), Use in Specific Populations (8.1, 8.4)].
In published studies, intraperitoneal administration of ketamine at doses greater than 40 mg/kg induced vacuolation in neuronal cells of the posterior cingulate and retrosplenial cortices in adult rats, similar to what has been reported in rodents administered other NMDA receptor antagonists. These vacuoles were demonstrated to be reversible and did not progress to degeneration or neuronal death up to doses of 80 mg/kg (1.2 times the human dose of 10 mg/kg based on body surface area). A no-effect level for neuronal vacuolation was 20 mg/kg intraperitoneal (0.3 times a human dose of 10 mg/kg on a body surface area basis). The window of vulnerability to these changes is believed to correlate with exposures in humans from the onset of puberty through adulthood. The relevance of this finding to humans is unknown.
Clinical Studies
14 CLINICAL STUDIES
Ketamine Hydrochloride Injection has been studied in over 12,000 operative and diagnostic procedures, involving over 10,000 patients in 105 separate studies. During the course of these studies, Ketamine Hydrochloride Injection was administered as the sole general anesthetic, as an induction agent prior to administration of other general anesthetics, or to supplement other anesthetic agents.
Ketamine Hydrochloride Injection has been evaluated during the following procedures:
- 1.
- debridement, dressing changes, and skin grafting in burn patients, as well as other superficial surgical procedures.
- 2.
- neurodiagnostic procedures such as myelograms and lumbar punctures.
- 3.
- diagnostic and operative procedures of the ear, nose, and mouth, including dental extractions.
- 4.
- sigmoidoscopy and minor surgery of the anus and rectum, and circumcision.
- 5.
- extraperitoneal procedures, such as dilatation and curettage.
- 6.
- orthopedic procedures such as closed reductions, manipulations, femoral pinning, amputations, and biopsies.
- 7.
- cardiac catheterization procedures.
How Supplied/Storage and Handling
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Ketamine Hydrochloride Injection, USP is a clear colorless solution supplied as the hydrochloride in concentrations equivalent to ketamine base.
| Unit of Sale | Concentration |
|---|---|
NDC 0409-2051-05 | 500 mg/5 mL |
NDC 0409-2053-10 | 500 mg/10 mL |
Medication Guide
17 PATIENT COUNSELING INFORMATION
- •
- Studies conducted in young animals and children suggest repeated or prolonged use of general anesthetic or sedation drugs in children younger than 3 years may have negative effects on their developing brains. Discuss with parents and caregivers the benefits, risks, and timing and duration of surgery or procedures requiring anesthetic and sedation drugs [see Warnings and Precautions (5.5)].
- •
- Due to the residual anesthetic effects and the potential for drowsiness, advise patients not to drive an automobile, operate hazardous machinery, or engage in hazardous activities within 24 hours of receiving Ketamine Hydrochloride Injection.
Other
17 PATIENT COUNSELING INFORMATION
17 PATIENT COUNSELING INFORMATION
- •
- Studies conducted in young animals and children suggest repeated or prolonged use of general anesthetic or sedation drugs in children younger than 3 years may have negative effects on their developing brains. Discuss with parents and caregivers the benefits, risks, and timing and duration of surgery or procedures requiring anesthetic and sedation drugs [see Warnings and Precautions (5.5)].
- •
- Due to the residual anesthetic effects and the potential for drowsiness, advise patients not to drive an automobile, operate hazardous machinery, or engage in hazardous activities within 24 hours of receiving Ketamine Hydrochloride Injection.
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
- •
- Studies conducted in young animals and children suggest repeated or prolonged use of general anesthetic or sedation drugs in children younger than 3 years may have negative effects on their developing brains. Discuss with parents and caregivers the benefits, risks, and timing and duration of surgery or procedures requiring anesthetic and sedation drugs [see Warnings and Precautions (5.5)].
- •
- Due to the residual anesthetic effects and the potential for drowsiness, advise patients not to drive an automobile, operate hazardous machinery, or engage in hazardous activities within 24 hours of receiving Ketamine Hydrochloride Injection.
Resources
Didn’t find what you were looking for?
Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this product, click the link below to submit your information: Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer product, please call Pfizer Medical Information at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800)-332-1088.