HEXTEND Description
(6% Hetastarch in Lactated Electrolyte Injection)
HEXTEND (6% Hetastarch in Lactated Electrolyte Injection) is a sterile, nonpyrogenic solution for intravenous administration. The composition, pH, and osmolarity are given in Table 1 and the electrolyte composition in Table 2.
Each 100 mL contains | |
Hetastarch | 6 g |
Sodium Chloride, USP | 672 mg |
Sodium Lactate Anhydrous, USP | 317 mg |
Dextrose Hydrous, USP | 99 mg |
Calcium Chloride Dihydrate, USP | 37 mg |
Potassium Chloride, USP | 22 mg |
Magnesium Chloride Hexahydrate, USP | 9 mg |
Water for Injection, USP | qs |
pH: approximately 5.9 with negligible buffering capacity | |
Calculated Osmolarity: approximately 307 mOsM | |
Concentration of Electrolytes (mEq/L) | |
Sodium | 143 |
Chloride | 124 |
Lactate | 28 |
Calcium | 5 |
Potassium | 3 |
Magnesium | 0.9 |
HEXTEND (6% Hetastarch in Lactated Electrolyte Injection) is an artificial colloidal solution, pharmacologically classified as a plasma volume expander, and is intended to support oncotic pressure as well as provide electrolytes.
HEXTEND contains high molecular weight hetastarch at a concentration of 6% as an oncotic agent to permit retention of intravascular fluid until the hetastarch is replaced by blood proteins. Hetastarch is an artificial colloid derived from a waxy starch composed almost entirely of amylopectin. Hydroxyethyl ether groups are introduced into the glucose units of the starch, and the resultant material is hydrolyzed to yield a product with a molecular weight suitable for use as a plasma volume expander. Hetastarch is characterized by its molar substitution and also by its molecular weight. The molar substitution is approximately 0.75 which means hetastarch has an average of approximately 75 hydroxyethyl groups for every 100 glucose units. The weight average molecular weight is approximately 670,000 with a range of 450,000 to 800,000 and with at least 80% of the polymer units falling within the range of 20,000 to 2,500,000. Hydroxyethyl groups are attached by ether linkage primarily at C-2 of the glucose unit and to a lesser extent at C-3 and C-6. The polymer resembles glycogen, and the polymerized D-glucose units are joined primarily by α-1,4 linkages with occasional α-1,6 branching linkages. The degree of branching is approximately 1:20 which means that there is an average of approximately one α-1,6 branch for every 20 glucose monomer units.
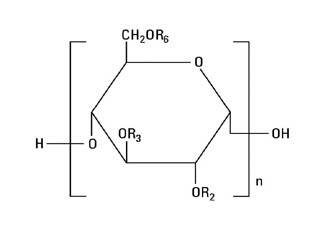
The chemical name for hetastarch is hydroxyethyl starch.
The structural formula is as follows:
Amylopectin derivative in which R2 and R3 are H or CH2CH2OH and R6 is H, CH2CH2OH, or a branching point in the starch polymer connected through an α-1,6 link to additional D-glucopyranosyl units
HEXTEND is a clear, pale yellow to amber solution. Exposure to prolonged adverse storage conditions may result in a change to a turbid deep brown or the formation of a crystalline precipitate. Do not use the solution if these conditions are evident.
HEXTEND is formulated with normal physiological levels of calcium. Calcium chloride in water dissociates to provide calcium (Ca++) and chloride (Cl-) ions. These are the normal constituents of the body fluids and are dependent on various physiologic mechanisms for maintenance of balance between intake and output. Approximately 80% of body calcium is excreted in the feces as insoluble salts; urinary excretion accounts for the remaining 20%.
HEXTEND also contains normal physiological levels of sodium. Additionally, chloride is present at levels closer to those normally found in blood than in plasma expanders in 0.9% Sodium Chloride Injection. Sodium chloride in water dissociates to provide sodium (Na+) and chloride (Cl-) ions. Sodium (Na+) is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Chloride (Cl-) has an integral role in buffering action when oxygen and carbon dioxide exchange occurs in the red blood cells. The distribution and excretion of sodium (Na+) and chloride (Cl-) are largely under the control of the kidneys, which maintain a balance between intake and output.
HEXTEND also contains slightly lower than normal physiological levels of potassium and magnesium. Potassium chloride in water dissociates to provide potassium (K+) and chloride (Cl-) ions. Potassium is found in low concentrations in plasma and extracellular fluids (3.5 to 5.0 mEq/L in a healthy adult). It is the most abundant cation within the body's cells (160 mEq/L of intracellular water). Potassium plays an important role in electrolyte balance. Normally about 80 to 90% of the potassium intake is excreted in the urine with the remainder in the stools and, to a small extent, in the perspiration. The kidney does not conserve potassium well so that during fasting or in patients on a potassium-free diet, potassium loss from the body continues thereby resulting in potassium depletion.
Magnesium chloride in water dissociates to provide magnesium (Mg++) and chloride (Cl-). Magnesium is largely an intracellular ion with low concentrations (1.5 to 2.5 mEq/L) found in plasma.
Dextrose is included in the formulation. Solutions containing carbohydrate in the form of dextrose yield blood glucose, provide calories, and may also aid in minimizing liver glycogen depletion thereby exerting a protein sparing effect. Dextrose injected parenterally undergoes oxidation to carbon dioxide and water.
Lactate is provided at 28 mEq/L. Sodium lactate in water dissociates to provide sodium (Na+) and lactate (C3H5O3-) ions. The lactate anion provides an alkaline effect resulting from simultaneous removal of lactate and hydrogen ions by the liver. In the liver, the lactate is metabolized to glycogen, which is ultimately converted to carbon dioxide and water by oxidative metabolism.
The lactate anion acts as a source (alternate) of bicarbonate when normal production and utilization of lactic acid is not impaired as a result of disordered lactate metabolism. Since metabolic conversion is dependent on the integrity of cellular oxidative processes, lactate may be inadequate or ineffective as a source of bicarbonate in patients suffering from acidosis associated with shock or other disorders involving reduced perfusion of body tissues. When oxidative activity is intact, one to two hours is required for metabolism of lactate.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. The average normal adult daily requirement ranges from 2 to 3 L (1.0 to 1.5 L each for insensible water loss by perspiration and urine production). Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments, and sodium (Na+) plays a major role in maintaining physiological equilibrium.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. The container solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
The closure system has two ports; the one for the administration set has a tamper evident plastic protector.
Find HEXTEND medical information:
Find HEXTEND medical information:
HEXTEND Quick Finder
Health Professional Information
Description
HEXTEND (6% Hetastarch in Lactated Electrolyte Injection) is a sterile, nonpyrogenic solution for intravenous administration. The composition, pH, and osmolarity are given in Table 1 and the electrolyte composition in Table 2.
Each 100 mL contains | |
Hetastarch | 6 g |
Sodium Chloride, USP | 672 mg |
Sodium Lactate Anhydrous, USP | 317 mg |
Dextrose Hydrous, USP | 99 mg |
Calcium Chloride Dihydrate, USP | 37 mg |
Potassium Chloride, USP | 22 mg |
Magnesium Chloride Hexahydrate, USP | 9 mg |
Water for Injection, USP | qs |
pH: approximately 5.9 with negligible buffering capacity | |
Calculated Osmolarity: approximately 307 mOsM | |
Concentration of Electrolytes (mEq/L) | |
Sodium | 143 |
Chloride | 124 |
Lactate | 28 |
Calcium | 5 |
Potassium | 3 |
Magnesium | 0.9 |
HEXTEND (6% Hetastarch in Lactated Electrolyte Injection) is an artificial colloidal solution, pharmacologically classified as a plasma volume expander, and is intended to support oncotic pressure as well as provide electrolytes.
HEXTEND contains high molecular weight hetastarch at a concentration of 6% as an oncotic agent to permit retention of intravascular fluid until the hetastarch is replaced by blood proteins. Hetastarch is an artificial colloid derived from a waxy starch composed almost entirely of amylopectin. Hydroxyethyl ether groups are introduced into the glucose units of the starch, and the resultant material is hydrolyzed to yield a product with a molecular weight suitable for use as a plasma volume expander. Hetastarch is characterized by its molar substitution and also by its molecular weight. The molar substitution is approximately 0.75 which means hetastarch has an average of approximately 75 hydroxyethyl groups for every 100 glucose units. The weight average molecular weight is approximately 670,000 with a range of 450,000 to 800,000 and with at least 80% of the polymer units falling within the range of 20,000 to 2,500,000. Hydroxyethyl groups are attached by ether linkage primarily at C-2 of the glucose unit and to a lesser extent at C-3 and C-6. The polymer resembles glycogen, and the polymerized D-glucose units are joined primarily by α-1,4 linkages with occasional α-1,6 branching linkages. The degree of branching is approximately 1:20 which means that there is an average of approximately one α-1,6 branch for every 20 glucose monomer units.
The chemical name for hetastarch is hydroxyethyl starch.
The structural formula is as follows:
Amylopectin derivative in which R2 and R3 are H or CH2CH2OH and R6 is H, CH2CH2OH, or a branching point in the starch polymer connected through an α-1,6 link to additional D-glucopyranosyl units
HEXTEND is a clear, pale yellow to amber solution. Exposure to prolonged adverse storage conditions may result in a change to a turbid deep brown or the formation of a crystalline precipitate. Do not use the solution if these conditions are evident.
HEXTEND is formulated with normal physiological levels of calcium. Calcium chloride in water dissociates to provide calcium (Ca++) and chloride (Cl-) ions. These are the normal constituents of the body fluids and are dependent on various physiologic mechanisms for maintenance of balance between intake and output. Approximately 80% of body calcium is excreted in the feces as insoluble salts; urinary excretion accounts for the remaining 20%.
HEXTEND also contains normal physiological levels of sodium. Additionally, chloride is present at levels closer to those normally found in blood than in plasma expanders in 0.9% Sodium Chloride Injection. Sodium chloride in water dissociates to provide sodium (Na+) and chloride (Cl-) ions. Sodium (Na+) is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Chloride (Cl-) has an integral role in buffering action when oxygen and carbon dioxide exchange occurs in the red blood cells. The distribution and excretion of sodium (Na+) and chloride (Cl-) are largely under the control of the kidneys, which maintain a balance between intake and output.
HEXTEND also contains slightly lower than normal physiological levels of potassium and magnesium. Potassium chloride in water dissociates to provide potassium (K+) and chloride (Cl-) ions. Potassium is found in low concentrations in plasma and extracellular fluids (3.5 to 5.0 mEq/L in a healthy adult). It is the most abundant cation within the body's cells (160 mEq/L of intracellular water). Potassium plays an important role in electrolyte balance. Normally about 80 to 90% of the potassium intake is excreted in the urine with the remainder in the stools and, to a small extent, in the perspiration. The kidney does not conserve potassium well so that during fasting or in patients on a potassium-free diet, potassium loss from the body continues thereby resulting in potassium depletion.
Magnesium chloride in water dissociates to provide magnesium (Mg++) and chloride (Cl-). Magnesium is largely an intracellular ion with low concentrations (1.5 to 2.5 mEq/L) found in plasma.
Dextrose is included in the formulation. Solutions containing carbohydrate in the form of dextrose yield blood glucose, provide calories, and may also aid in minimizing liver glycogen depletion thereby exerting a protein sparing effect. Dextrose injected parenterally undergoes oxidation to carbon dioxide and water.
Lactate is provided at 28 mEq/L. Sodium lactate in water dissociates to provide sodium (Na+) and lactate (C3H5O3-) ions. The lactate anion provides an alkaline effect resulting from simultaneous removal of lactate and hydrogen ions by the liver. In the liver, the lactate is metabolized to glycogen, which is ultimately converted to carbon dioxide and water by oxidative metabolism.
The lactate anion acts as a source (alternate) of bicarbonate when normal production and utilization of lactic acid is not impaired as a result of disordered lactate metabolism. Since metabolic conversion is dependent on the integrity of cellular oxidative processes, lactate may be inadequate or ineffective as a source of bicarbonate in patients suffering from acidosis associated with shock or other disorders involving reduced perfusion of body tissues. When oxidative activity is intact, one to two hours is required for metabolism of lactate.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. The average normal adult daily requirement ranges from 2 to 3 L (1.0 to 1.5 L each for insensible water loss by perspiration and urine production). Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments, and sodium (Na+) plays a major role in maintaining physiological equilibrium.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. The container solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
The closure system has two ports; the one for the administration set has a tamper evident plastic protector.
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.