HEXTEND
(6% Hetastarch in Lactated Electrolyte Injection)
Find HEXTEND medical information:
Find HEXTEND medical information:
HEXTEND Quick Finder
Boxed Warning
Indications and Usage
Dosage and Administration
DOSAGE AND ADMINISTRATION
Dosage for Acute Use in Plasma Volume Expansion
HEXTEND is administered by intravenous infusion only. Total dosage and rate of infusion depend upon the amount of blood or plasma lost and the resultant hemoconcentration as well as age, weight, and clinical condition of the patient.
Adults: The amount usually administered is 500 to 1000 mL. Doses of more than 1500 mL per day for the typical 70 kg patient (approximately 20 mL per kg of body weight) are usually not required although doses of isotonic solutions containing 6% hetastarch up to 1500 mL have been used during major surgery generally without a need for blood or blood products. Volumes in excess of 1500 mL per day have been used where severe blood loss has occurred although generally only in conjunction with the administration of blood and blood products (see WARNINGS AND PRECAUTIONS).
Pediatric Patients: Adequate, well controlled clinical trials to establish the safety and effectiveness of HEXTEND in pediatric patients have not been conducted (see WARNINGS AND PRECAUTIONS, Pediatric Use).
General Recommendations
Do not use plastic container in series connection.
If administration is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result.
This solution is intended for intravenous administration using sterile equipment. It is recommended that intravenous administration apparatus be replaced at least once every 24 hours.
Use only if solution is clear and container and seals are intact.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
If administration is by pressure infusion, all air should be withdrawn or expelled from the bag through the medication port prior to infusion.
The safety and compatibility of other additives have not been established. This product contains calcium and should not be administered simultaneously with blood through the same administration set because of the likelihood of coagulation (see WARNINGS AND PRECAUTIONS).
The solution contains no bacteriostat or antimicrobial agent and is intended only for single-dose injection. When smaller doses are required, the unused portion should be discarded.
Contraindications
Warnings and Precautions
WARNINGS AND PRECAUTIONS
- Increased risk of mortality and acute kidney injury (AKI) in critically ill patients, including patients with sepsis; surgical patients; and blunt trauma patients
- Avoid use in patients with pre-existing renal dysfunction
- Discontinue use of HEXTEND at the first sign of renal injury
- Continue to monitor renal function for at least 90 days as use of renal replacement therapy (RRT) has been reported up to 90 days after administration of HES products
- Monitor the coagulation status of surgery patients, as excess bleeding has been reported with HES solutions in this population. Discontinue use of HEXTEND at the first sign of coagulopathy
- Monitor liver function in patients receiving HES products, including HEXTEND
Solutions containing calcium should not be administered simultaneously with blood through the same administration set because of the likelihood of coagulation.
Life threatening anaphylactic/anaphylactoid reactions have been rarely reported with solutions containing hetastarch; death has occurred, but a causal relationship has not been established. Patients who develop severe anaphylactic/anaphylactoid reactions may need continued supportive care until symptoms have resolved.
Hypersensitivity reactions can occur even after solutions containing hetastarch have been discontinued.
Solutions which contain potassium should be used with great care, if at all, in patients with hyperkalemia and severe renal failure and in situations in which potassium retention is present.
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure and severe renal insufficiency and in clinical states in which edema with sodium retention occurs.
In patients with diminished renal function, administration of solutions containing sodium or potassium ions may result in sodium or potassium retention.
Solutions containing lactate ions should be used with great care in patients with metabolic or respiratory alkalosis. The administration of lactate ions should be performed with great care when dealing with conditions in which an increased level or an impaired utilization of these ions occurs, such as severe hepatic insufficiency.
DO NOT USE IN LEUKAPHERESIS.
Usage in Plasma Volume Expansion
Large volumes of isotonic solutions containing 6% hetastarch (HEXTEND or 6% Hetastarch in 0.9% Sodium Chloride Injection) may transiently alter the coagulation mechanism due to hemodilution and a direct inhibitory action on Factor VIII. Hemodilution by isotonic solutions containing 6% hetastarch may also result in a 24 hour decline of total protein, albumin, and fibrinogen levels and in transient prolongation of prothrombin, activated partial thromboplastin, clotting, and bleeding times.
Increased bleeding has been reported in patients undergoing open heart surgery in association with cardiopulmonary bypass following the use of 6% Hetastarch in 0.9% Sodium Chloride Injection.5,6 Although the electrolyte content of HEXTEND resembles that of the principal ionic constituents of normal plasma, its 6% Hetastarch component is identical to that of 6% Hetastarch in 0.9% Sodium Chloride Injection and has a direct inhibitory action on Factor VIII. There are no retrospective studies similar to those with 6% Hetastarch in 0.9% Sodium Chloride Injection that compare HEXTEND to albumin in cardiopulmonary bypass surgery nor have any adequate and well-controlled clinical studies been performed.
Hematocrit may be decreased and plasma proteins diluted excessively by administration of large volumes of isotonic solutions containing 6% hetastarch. Administration of packed red cells, platelets, and fresh frozen plasma should be considered if excessive dilution occurs.
In randomized, controlled, comparative studies of 6% Hetastarch in 0.9% Sodium Chloride Injection (n = 92) and Albumin (n = 85) in surgical patients, no patient in either treatment group had a bleeding complication and no significant difference was found in the amount of blood loss between the treatment groups.1-4
HEXTEND has not been adequately evaluated to establish its safety in situations other than treatment of hypovolemia in elective surgery. In some cases, the use of isotonic solutions containing 6% hetastarch has been associated with coagulation abnormalities in conjunction with an acquired, reversible von Willebrand's-like syndrome and/or Factor VIII deficiency when used over a period of days. Replacement therapy should be considered if a severe Factor VIII or von Willebrand deficiency is identified. If a coagulopathy develops, it may take several days to resolve. Certain conditions may affect the safe use of isotonic solutions containing 6% hetastarch on a chronic basis. For example, in patients with subarachnoid hemorrhage where an isotonic solution containing 6% hetastarch is used repeatedly over a period of days for the prevention of cerebral vasospasm, significant clinical bleeding may occur. Intracranial bleeding resulting in death has been reported with the use of 6% Hetastarch in 0.9% Sodium Chloride Injection.7
The possibility of circulatory overload should be kept in mind. Caution should be used when the risk of pulmonary edema and/or congestive heart failure is increased. Special care should be exercised in patients who have impaired renal clearance since this is the principal way in which hetastarch is eliminated and in clinical states in which edema with sodium retention occurs.
Indirect bilirubin levels of 8.3 mg/L (normal 0.0-7.0 mg/L) have been reported in 2 out of 20 normal subjects who received multiple infusions of 6% Hetastarch in 0.9% Sodium Chloride Injection. Total bilirubin was within normal limits at all times; indirect bilirubin returned to normal by 96 hours following the final infusion. The significance, if any, of these elevations is not known; however, caution should be observed before administering isotonic solutions containing 6% hetastarch to patients with a history of liver disease.
If a hypersensitivity effect occurs, administration of the drug should be discontinued and appropriate treatment and supportive measures should be undertaken (see WARNINGS AND PRECAUTIONS).
Caution should be used when administering solutions containing hetastarch to patients allergic to corn because such patients can also be allergic to hetastarch.
HEXTEND should be used with caution in patients who have been anticoagulated with other drugs that negatively influence the coagulation system.
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, acid-base balance, and coagulation parameters during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
Potassium containing solutions should be used with caution in the presence of cardiac disease, particularly in digitalized patients or in the presence of renal disease.
Solutions containing lactate ions should be used with caution as excess administration may result in metabolic alkalosis.
Elevated serum amylase levels may be observed temporarily following administration of solutions containing hetastarch although no association with pancreatitis has been demonstrated. Serum amylase levels cannot be used to assess or to evaluate for pancreatitis for 3-5 days after administration of solutions containing hetastarch. Elevated serum amylase levels persist for longer periods of time in patients with renal impairment. Solutions containing hetastarch have not been shown to increase serum lipase.
One report suggests that in the presence of renal glomerular damage, larger molecules of hetastarch can leak into the urine and elevate the specific gravity. The elevation of specific gravity can obscure the diagnosis of renal failure.
Hetastarch is not eliminated by hemodialysis. The utility of other extracorporeal elimination techniques has not been evaluated.
If administration is by pressure infusion, all air should be withdrawn or expelled from the bag through the medication port prior to infusion.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies of animals have not been performed to evaluate the carcinogenic potential of hetastarch.
Teratogenic Effects: Pregnancy Category C.
6% Hetastarch in 0.9% Sodium Chloride Injection has been shown to have an embryocidal effect on New Zealand rabbits when given intravenously over the entire organogenesis period in a daily dose 1/2 times the maximum recommended therapeutic human dose (1500 mL) and on BD rats when given intraperitoneally, from the 16th to the 21st day of pregnancy, in a daily dose 2.3 times the maximum recommended therapeutic human dose. When 6% Hetastarch in 0.9% Sodium Chloride Injection was administered to New Zealand rabbits, BD rats, and Swiss mice with intravenous daily doses of 2 times, 1/3 times, and 1 times the maximum recommended therapeutic human dose, respectively, over several days during the period of gestation, no evidence of teratogenicity was evident. There are no adequate and well controlled studies in pregnant women. HEXTEND should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether hetastarch is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when HEXTEND is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of HEXTEND in pediatric patients have not been established. Adequate, well-controlled clinical trials to establish the safety and effectiveness of HEXTEND in pediatric patients have not been conducted. However, in one small double-blind study, 47 infants, children, and adolescents (ages 1 year to 15.5 years) scheduled for repair of congenital heart disease with moderate hypothermia were randomized to receive either 6% Hetastarch in 0.9% Sodium Chloride Injection or Albumin as a postoperative volume expander during the first 24 hours after surgery. Thirty-eight children required colloid replacement therapy, of which 20 children received 6% Hetastarch in 0.9% Sodium Chloride Injection. No differences were found in the coagulation parameters or in the amount of replacement fluids required in the children receiving 20 mL/kg or less of either colloid replacement therapy. In children who received greater than 20 mL/kg of 6% Hetastarch in 0.9% Sodium Chloride Injection, an increase in prothrombin time was demonstrated (p = 0.006).8 There were no neonates included in this study.
Geriatric Use
Of the total number of patients in clinical trials of HEXTEND (n=119), 30% were 65 or older while 12% were 70 or older. Other reported experience with 6% Hetastarch in 0.9% Sodium Chloride Injection has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Adverse Reactions
ADVERSE REACTIONS
Serious adverse reactions reported in postmarketing clinical trials include increased mortality and AKI (including need for RRT) in critically ill subjects, including subjects with sepsis, and surgical subjects. Clinical trials have also shown increased mortality and AKI in blunt trauma subjects. Increased coagulopathy was reported in surgical subjects.
In clinical trials comparing the plasma volume expanding properties of HEXTEND (n=60) with those of 6% Hetastarch in 0.9% Sodium Chloride Injection (n=59), there were no significant differences in the number of adverse or serious adverse events between the two groups.
Reported adverse reactions with isotonic solutions containing 6% hetastarch include:
General
Hypersensitivity (see WARNINGS AND PRECAUTIONS).
Death, life-threatening anaphylactic/anaphylactoid reactions, cardiac arrest, ventricular fibrillation, severe hypotension, non-cardiac pulmonary edema, laryngeal edema, bronchospasm, angioedema, wheezing, restlessness, tachypnea, stridor, fever, chest pain, bradycardia, tachycardia, shortness of breath, chills, urticaria, pruritus, facial and periorbital edema, coughing, sneezing, flushing, erythema multiforme, and rash.
Cardiovascular
Circulatory overload, congestive heart failure, and pulmonary edema (see WARNINGS AND PRECAUTIONS).
Hematologic
Intracranial bleeding, bleeding and/or anemia due to hemodilution (see WARNINGS AND PRECAUTIONS) and/or Factor VIII deficiency, acquired von Willebrand's-like syndrome, and coagulopathy including rare cases of disseminated intravascular coagulopathy and hemolysis. With extensive clinical use of 6% Hetastarch in 0.9% Sodium Chloride Injection, rare cases of disseminated intravascular coagulopathy and hemolysis have been observed.
Metabolic
Metabolic acidosis.
Other
Vomiting, peripheral edema of the lower extremities, submaxillary and parotid glandular enlargement, mild influenza-like symptoms, headaches, and muscle pains. Hydroxyethyl starch-associated pruritus has been reported in some patients with deposits of hydroxyethyl starch in peripheral nerves.
Description
HEXTEND (6% Hetastarch in Lactated Electrolyte Injection) is a sterile, nonpyrogenic solution for intravenous administration. The composition, pH, and osmolarity are given in Table 1 and the electrolyte composition in Table 2.
Each 100 mL contains | |
Hetastarch | 6 g |
Sodium Chloride, USP | 672 mg |
Sodium Lactate Anhydrous, USP | 317 mg |
Dextrose Hydrous, USP | 99 mg |
Calcium Chloride Dihydrate, USP | 37 mg |
Potassium Chloride, USP | 22 mg |
Magnesium Chloride Hexahydrate, USP | 9 mg |
Water for Injection, USP | qs |
pH: approximately 5.9 with negligible buffering capacity | |
Calculated Osmolarity: approximately 307 mOsM | |
Concentration of Electrolytes (mEq/L) | |
Sodium | 143 |
Chloride | 124 |
Lactate | 28 |
Calcium | 5 |
Potassium | 3 |
Magnesium | 0.9 |
HEXTEND (6% Hetastarch in Lactated Electrolyte Injection) is an artificial colloidal solution, pharmacologically classified as a plasma volume expander, and is intended to support oncotic pressure as well as provide electrolytes.
HEXTEND contains high molecular weight hetastarch at a concentration of 6% as an oncotic agent to permit retention of intravascular fluid until the hetastarch is replaced by blood proteins. Hetastarch is an artificial colloid derived from a waxy starch composed almost entirely of amylopectin. Hydroxyethyl ether groups are introduced into the glucose units of the starch, and the resultant material is hydrolyzed to yield a product with a molecular weight suitable for use as a plasma volume expander. Hetastarch is characterized by its molar substitution and also by its molecular weight. The molar substitution is approximately 0.75 which means hetastarch has an average of approximately 75 hydroxyethyl groups for every 100 glucose units. The weight average molecular weight is approximately 670,000 with a range of 450,000 to 800,000 and with at least 80% of the polymer units falling within the range of 20,000 to 2,500,000. Hydroxyethyl groups are attached by ether linkage primarily at C-2 of the glucose unit and to a lesser extent at C-3 and C-6. The polymer resembles glycogen, and the polymerized D-glucose units are joined primarily by α-1,4 linkages with occasional α-1,6 branching linkages. The degree of branching is approximately 1:20 which means that there is an average of approximately one α-1,6 branch for every 20 glucose monomer units.
The chemical name for hetastarch is hydroxyethyl starch.
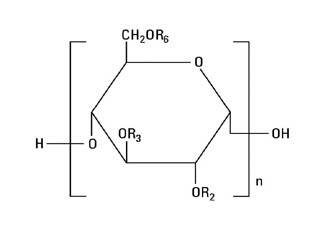
The structural formula is as follows:
Amylopectin derivative in which R2 and R3 are H or CH2CH2OH and R6 is H, CH2CH2OH, or a branching point in the starch polymer connected through an α-1,6 link to additional D-glucopyranosyl units
HEXTEND is a clear, pale yellow to amber solution. Exposure to prolonged adverse storage conditions may result in a change to a turbid deep brown or the formation of a crystalline precipitate. Do not use the solution if these conditions are evident.
HEXTEND is formulated with normal physiological levels of calcium. Calcium chloride in water dissociates to provide calcium (Ca++) and chloride (Cl-) ions. These are the normal constituents of the body fluids and are dependent on various physiologic mechanisms for maintenance of balance between intake and output. Approximately 80% of body calcium is excreted in the feces as insoluble salts; urinary excretion accounts for the remaining 20%.
HEXTEND also contains normal physiological levels of sodium. Additionally, chloride is present at levels closer to those normally found in blood than in plasma expanders in 0.9% Sodium Chloride Injection. Sodium chloride in water dissociates to provide sodium (Na+) and chloride (Cl-) ions. Sodium (Na+) is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Chloride (Cl-) has an integral role in buffering action when oxygen and carbon dioxide exchange occurs in the red blood cells. The distribution and excretion of sodium (Na+) and chloride (Cl-) are largely under the control of the kidneys, which maintain a balance between intake and output.
HEXTEND also contains slightly lower than normal physiological levels of potassium and magnesium. Potassium chloride in water dissociates to provide potassium (K+) and chloride (Cl-) ions. Potassium is found in low concentrations in plasma and extracellular fluids (3.5 to 5.0 mEq/L in a healthy adult). It is the most abundant cation within the body's cells (160 mEq/L of intracellular water). Potassium plays an important role in electrolyte balance. Normally about 80 to 90% of the potassium intake is excreted in the urine with the remainder in the stools and, to a small extent, in the perspiration. The kidney does not conserve potassium well so that during fasting or in patients on a potassium-free diet, potassium loss from the body continues thereby resulting in potassium depletion.
Magnesium chloride in water dissociates to provide magnesium (Mg++) and chloride (Cl-). Magnesium is largely an intracellular ion with low concentrations (1.5 to 2.5 mEq/L) found in plasma.
Dextrose is included in the formulation. Solutions containing carbohydrate in the form of dextrose yield blood glucose, provide calories, and may also aid in minimizing liver glycogen depletion thereby exerting a protein sparing effect. Dextrose injected parenterally undergoes oxidation to carbon dioxide and water.
Lactate is provided at 28 mEq/L. Sodium lactate in water dissociates to provide sodium (Na+) and lactate (C3H5O3-) ions. The lactate anion provides an alkaline effect resulting from simultaneous removal of lactate and hydrogen ions by the liver. In the liver, the lactate is metabolized to glycogen, which is ultimately converted to carbon dioxide and water by oxidative metabolism.
The lactate anion acts as a source (alternate) of bicarbonate when normal production and utilization of lactic acid is not impaired as a result of disordered lactate metabolism. Since metabolic conversion is dependent on the integrity of cellular oxidative processes, lactate may be inadequate or ineffective as a source of bicarbonate in patients suffering from acidosis associated with shock or other disorders involving reduced perfusion of body tissues. When oxidative activity is intact, one to two hours is required for metabolism of lactate.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. The average normal adult daily requirement ranges from 2 to 3 L (1.0 to 1.5 L each for insensible water loss by perspiration and urine production). Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments, and sodium (Na+) plays a major role in maintaining physiological equilibrium.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. The container solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
The closure system has two ports; the one for the administration set has a tamper evident plastic protector.
Clinical Pharmacology
CLINICAL PHARMACOLOGY
The plasma volume expansion produced by HEXTEND approximates that produced by 6% Hetastarch in 0.9% Sodium Chloride Injection and the plasma volume expansion of 6% Hetastarch in 0.9% Sodium Chloride Injection in turn approximates that of 5% Albumin (Human).1-4 In randomized, double-blind studies of HEXTEND versus control (6% Hetastarch in 0.9% Sodium Chloride Injection) for the treatment of hypovolemia in elective surgery, 60 patients were infused with a mean of 1596 mL of HEXTEND and 59 patients were infused with a mean of 1428 mL of 6% Hetastarch in 0.9% Sodium Chloride Injection without any serious related adverse events. 59% of the HEXTEND patients and 59% of the control patients required packed red blood cells and other blood-derived products either intraoperatively or postoperatively.
Intravenous infusion of 6% Hetastarch in 0.9% Sodium Chloride Injection results in expansion of plasma volume that decreases over the succeeding 24 to 36 hours. The degree of plasma volume expansion and improvement in hemodynamic state depend upon the patient's intravascular status. When administered intravenously, HEXTEND provides sources of water and electrolytes. Its electrolyte content resembles that of the principal ionic constituents of normal plasma.
Hetastarch molecules below 50,000 molecular weight are rapidly eliminated by renal excretion. A single dose of approximately 500 mL of 6% Hetastarch in 0.9% Sodium Chloride Injection (approximately 30 g) results in elimination in the urine of approximately 33% of the dose within 24 hours. This is a variable process but generally results in an intravascular hetastarch concentration of less than 10% of the total dose injected by two weeks. A study of the biliary excretion of hetastarch in 10 healthy males accounted for less than 1% of the dose over a 14 day period. The hydroxyethyl group is not cleaved by the body but remains intact and attached to glucose units when excreted. Significant quantities of glucose are not produced as hydroxyethylation prevents complete metabolism of the smaller polymers.
Clinical Studies
CLINICAL STUDIES
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. (Note: All of the studies listed below used licensed HES products except for reference 9.)
A randomized controlled trial (N=804) in severe sepsis patients using an HES product not licensed in the U.S. reported increased mortality (relative risk, 1.17; 95% CI, 1.01 to 1.36; p=0.03) and RRT (relative risk, 1.35; 95% CI, 1.01 to 1.80; p=0.04) in the HES treatment arm.9
Another randomized controlled trial (N=196) in severe sepsis patients reported no difference in mortality (relative risk, 1.20; 95% CI, 0.83 to 1.74; p=0.33) and a trend for RRT (relative risk, 1.83; 95% CI, 0.93 to 3.59; p=0.06) in HES patients.10
A randomized controlled trial (N=7000) in a heterogeneous population of ICU patients reported no difference in mortality (relative risk, 1.06; 95% CI, 0.96 to 1.18; p=0.26) but increased use of RRT (relative risk, 1.21; 95% CI, 1.00 to 1.45; p=0.04 in HES patients.11
In a retrospective study of adult patients (N=1442) undergoing pulmonary or esophageal surgery who were prophylactically fluid-restricted during the procedure, 74 developed AKI (5.1%) within the first 72 hours postoperatively. Fluid restriction neither increased nor was a risk factor for AKI. AKI occurred more often when HES products were administered to patients with decreased renal function or having >2 risk factors with normal renal function, whereas restriction of crystalloid was unrelated to AKI, regardless of preoperative renal function.12
In a retrospective case series of high-risk adult vascular surgery patients (N=796) receiving fluid therapy during a vascular surgery procedure, logistic regression analysis using prespecified confounding variables or suspected risk factors for AKI that showed intraoperative administration of an HES product was associated with increased likelihood of 30-day mortality and need for RRT, compared with use of crystalloids alone.13
In a retrospective study of adult patients undergoing elective noncardiac surgery, patients (N=14,680) receiving an HES product and crystalloid were propensity-matched with patients (N=14,680) receiving only crystalloid. After controlling for potential confounding variables, odds of experiencing AKI of severe intensity with HES was 21% greater than with crystalloid alone. In addition, AKI risk increased as a function of HES volume.14
In a prospective observational study assessing the impact of HES products on recipient renal graft outcomes in brain-dead organ donors, data were obtained on 986 kidneys transplanted from 529 donors. Kidneys from donors who received HES had a higher rate of delayed graft function in recipient subjects (41% versus 31%). After accounting for the propensity of donors to receive HES products, HES product administration was independently associated with an increased risk of delayed graft function in recipients. A dose-response relationship also was evident.15
In a randomized, controlled trial of adult subjects (N=33) undergoing elective cystectomy comparing an HES product versus lactated Ringer's, administration of HES reduced clot strength (Maximum Amplitude; p<0.001) and increased blinded evaluation of perioperative blood loss by more than 50% (2181 mL versus 1370 mL, respectively; p=0.04). There was no significant between-group difference with respect to frequency of reoperation or length of hospital stay.16
In a prospective, sequential, observational study in adult subjects undergoing open heart surgery in association with cardiopulmonary bypass, fluid therapy using only an HES product (2004–2006, N=2137), 4% gelatin (2006–2008, N=2324) and crystalloids (N=2017, 2008–2010) led to increased need for renal replacement therapy after HES and gelatin, compared with crystalloid. Propensity score stratification confirmed greater use of RRT in the HES and gelatin periods compared to the crystalloid period. Fluid intake was higher in the crystalloid group only during the first 20 hours.17
In a retrospective observational study, 606 adult patients underwent open heart surgery in association with cardiopulmonary bypass. Until July 2013 they received an HES product (N=247) both as pump prime (1500 mL) and intraoperative fluid replacement (1000 mL), but only crystalloid (N=359) from August 2013 onward. The frequency (percent) of postoperative AKI was higher in patients receiving HES (N=53; 21.5%) than those receiving crystalloid (N=34; 9.5%). Surgical revision for rebleeding also was higher for HES (N=11; 4.6%) than for crystalloid (N=5; 1.4%).18
In a meta-analysis of RCTs (n=15) in adult subjects (N=4409) undergoing surgery who received an HES product, significantly more HES subjects (83/2157; 3.8%) than controls (56/2252; 2.5%) underwent RRT (relative risk, 1.44; 95% CI, 1.04, 2.01).19
In a retrospective observational study of adult blunt and penetrating trauma patients, use of an HES product was a significant independent predictor of AKI after blunt trauma, but not penetrating trauma, in multiple logistic regression analysis. In separate logistic regression models, HES also was a significant predictor of mortality after blunt trauma but not penetrating trauma.20
In a retrospective observational study of severely injured adult blunt (89%) and penetrating (11%) trauma patients (N=413) admitted to the ICU, 103 patients developed AKI within the first week of ICU admission. AKI was associated with increased 30-day (17.5% versus 5.8%, AKI versus non-AKI cohorts, respectively) and 1-year mortality (26.2% versus 7.1%). Univariate and multivariable regression analyses of prespecified risk factors for AKI found that volume loading using an HES product was independently associated with postinjury AKI within the first 24 hours.21
How Supplied/Storage and Handling
HOW SUPPLIED
HEXTEND (6% Hetastarch in Lactated Electrolyte Injection) is supplied sterile and nonpyrogenic in 500 mL single-dose flexible plastic infusion containers.
Unit of Sale | Volume/Container | Each |
NDC 0409-1555-54 Case containing 12 | 500 mL | NDC 0409-1555-64 Single-dose Flexible Container |
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. It is recommended that the product be stored at room temperature (25°C/77°F); however, brief exposure up to 40°C does not adversely affect the product.
References:
- 1.
- Diehl J, et al., Clinical Comparison of Hetastarch and Albumin in Postoperative Cardiac Patients. The Annals of Thoracic Surgery, 1982;34(6):674-679.
- 2.
- Gold M, et al., Comparison of Hetastarch to Albumin for Perioperative Bleeding in Patients Undergoing Abdominal Aortic Aneurysm Surgery, Annals of Surgery, 1990;211(4):482-485.
- 3.
- Kirklin J, et al., Hydroxyethyl Starch versus Albumin for Colloid Infusion Following Cardiopulmonary Bypass in Patients Undergoing Myocardial Revascularization, The Annals of Thoracic Surgery, 1984;37(1):40-46.
- 4.
- Moggio RA, et al., Hemodynamic Comparison of Albumin and Hydroxyethyl Starch in Postoperative Cardiac Surgery Patients, Critical Care Medicine, 1983;11(12):943-945.
- 5.
- Knutson, JE, et al., Does Intraoperative Hetastarch Administration Increase Blood Loss and Transfusion Requirements After Cardiac Surgery? Anesthesia Analg., 2000;90: 801-7.
- 6.
- Cope, JT, et al., Intraoperative Hetastarch Infusion Impairs Hemostasis After Cardiac Operations, The Annals of Thoracic Surgery, 1997;63: 78-83.
- 7.
- Damon L, Intracranial Bleeding During Treatment with Hydroxyethyl Starch, New England Journal of Medicine, 1987; 317(15):964-965.
- 8.
- Brutocao D, et al., Comparison of Hetastarch with Albumin for Postoperative Volume Expansion in Children After Cardiopulmonary Bypass, Journal of Cardiothoracic and Vascular Anesthesia, 1996;10(3):348-351.
- 9.
- Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl Starch 130/0.42 versus Ringer's Acetate in Severe Sepsis. N Eng J Med 2012; 367(2): 124-34
- 10.
- CRYSTMAS: Guidet B, Martinet O, Boulain T, et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 versus 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS study. Crit Care 2012; 16(3): R94
- 11.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367(20): 1901-11
- 12.
- Ahn HJ, Kim JA, Lee AR, et al. The risk of acute kidney injury from fluid restriction and hydroxyethyl starch in thoracic surgery. Anesth Analg 2016; 122(1):186–193.
- 13.
- Green RS, Butler MB, Hicks SD, et al. Effect of hydroxyethyl starch on outcomes in high-risk vascular surgery patients: A retrospective analysis. J Cardiothorac Vasc Anesth 2016; 30(4):967–72.
- 14.
- Kashy BK, Podolyak A, Makarova N, et al. Effect of hydroxyethyl starch on postoperative kidney function in patients having noncardiac surgery. Anesthesiology 2014; 121 (4):730–9.
- 15.
- Patel MS, Niemann CU, Sally MB, et al. The impact of hydroxyethyl starch use in deceased organ donors on the development of delayed graft function in kidney transplant recipients: A Propensity-Adjusted Analysis. Am J of Transplant 2015; 15 (8):2152–8.
- 16.
- Rasmussen KC, Johansson PI, Højskov M, et al. Hydroxyethyl starch reduces coagulation competence and increases blood loss during major surgery: results from a randomized controlled trial. Ann Surg 2014; 259 (2):249–54.
- 17.
- Bayer O, Schwarzkopf D, Doenst T, et al. Perioperative fluid therapy with tetrastarch and gelatin in cardiac surgery – a prospective sequential analysis. Crit Care Med 2013; 41(11):2532–42.
- 18.
- Lagny MG, Roediger L, Koch JN, et al. Hydroxyethyl starch 130/0.4 and the risk of acute kidney injury after cardiopulmonary bypass: A single-center retrospective study. J of Cardiothorac and Vasc Anesth 2016: 30(4):869–75.
- 19.
- Wilkes MM, Navickis RJ. Postoperative renal replacement therapy after hydroxyethyl starch infusion: a meta-analysis of randomized trials. Neth J Crit Care 2014; 18:4–9.
- 20.
- Allen CJ, Valle EJ, Jouria JM, et al. Differences between blunt and penetrating trauma after resuscitation with HES. J Trauma Acute Care Surg 2014; 77(6):859–64.
- 21.
- Eriksson M, Brattström O, Mårtensson J, et al. Acute kidney injury following severe trauma: Risk factors and long-term outcome. J Trauma Acute Care Surg 2015; 79(3):407–12.
To Open
Tear overwrap down at notch and remove solution container. Check for any leakage by squeezing solution container firmly. If leaks are found, discard solution as sterility may be impaired.
Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter. Any container that is suspect should not be used.
Distributed by: Hospira, Inc., Lake Forest, IL 60045 USA
LAB-1288-2.0
Revised: 10/2021
Other
Resources
Didn’t find what you were looking for?
Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this product, click the link below to submit your information: Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer product, please call Pfizer Medical Information at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800)-332-1088.