GLUCOTROL® XL
(glipizide extended release tablets)
Find GLUCOTROL® XL medical information:
Find GLUCOTROL® XL medical information:
GLUCOTROL® XL Quick Finder
Highlights
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GLUCOTROL XL safely and effectively. See full prescribing information for GLUCOTROL XL. GLUCOTROL XL (glipizide) extended release tablets, for oral use Initial U.S. Approval: 1994 INDICATIONS AND USAGEDOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSTablets: 2.5 mg, 5 mg, 10 mg (3). CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (incidence > 3%) are dizziness, diarrhea, nervousness, tremor, hypoglycemia and flatulence (6.1). To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 8/2023 |
Indications and Usage
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
GLUCOTROL XL should be administered orally with breakfast or the first main meal of the day.
The recommended starting dose of GLUCOTROL XL is 5 mg once daily. Start patients at increased risk for hypoglycemia (e.g. the elderly or patients with hepatic insufficiency) at 2.5 mg [see Use in Specific Population (8.5, 8.6)].
Dosage adjustment can be made based on the patient's glycemic control. The maximum recommended dose is 20 mg once daily.
Patients receiving immediate release glipizide may be switched to GLUCOTROL XL once daily at the nearest equivalent total daily dose.
2.2 Use with Other Glucose Lowering Agents
When adding GLUCOTROL XL to other anti-diabetic drugs, initiate GLUCOTROL XL at 5 mg once daily. Start patients at increased risk for hypoglycemia at a lower dose.
When colesevelam is coadministered with glipizide ER, maximum plasma concentration and total exposure to glipizide is reduced. Therefore, GLUCOTROL XL should be administered at least 4 hours prior to colesevelam.
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Hypoglycemia
All sulfonylurea drugs, including GLUCOTROL XL, are capable of producing severe hypoglycemia [see Adverse Reactions (6)]. Concomitant use of GLUCOTROL XL with other anti-diabetic medication can increase the risk of hypoglycemia. A lower dose of GLUCOTROL XL may be required to minimize the risk of hypoglycemia when combining it with other anti-diabetic medications.
Educate patients to recognize and manage hypoglycemia. When initiating and increasing GLUCOTROL XL in patients who may be predisposed to hypoglycemia (e.g., the elderly, patients with renal impairment, patients on other anti-diabetic medications) start at 2.5 mg. Debilitated or malnourished patients, and those with adrenal, pituitary, or hepatic impairment are particularly susceptible to the hypoglycemic action of anti-diabetic medications. Hypoglycemia is also more likely to occur when caloric intake is deficient, after severe or prolonged exercise, or when alcohol is ingested.
The patient's ability to concentrate and react may be impaired as a result of hypoglycemia. Early warning symptoms of hypoglycemia may be different or less pronounced in patients with autonomic neuropathy, the elderly, and in patients who are taking beta-adrenergic blocking medications or other sympatholytic agents. These situations may result in severe hypoglycemia before the patient is aware of the hypoglycemia.
These impairments may present a risk in situations where these abilities are especially important, such as driving or operating other machinery. Severe hypoglycemia can lead to unconsciousness or convulsions and may result in temporary or permanent impairment of brain function or death.
5.2 Hemolytic Anemia
Treatment of patients with glucose 6-phosphate dehydrogenase (G6PD) deficiency with sulfonylurea agents, including GLUCOTROL XL, can lead to hemolytic anemia. Avoid use of GLUCOTROL XL in patients with G6PD deficiency. In post marketing reports, hemolytic anemia has also been reported in patients who did not have known G6PD deficiency.
5.3 Increased Risk of Cardiovascular Mortality with Sulfonylureas
The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with type 2 diabetes mellitus. The study involved 823 patients who were randomly assigned to one of four treatment groups.
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2½ times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of glipizide and of alternative modes of therapy.
Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
5.4 Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with GLUCOTROL XL or any other anti-diabetic drug.
5.5 Gastrointestinal Obstruction
There have been reports of obstructive symptoms in patients with known strictures in association with the ingestion of another drug with this non-dissolvable extended release formulation. Avoid use of GLUCOTROL XL in patients with preexisting severe gastrointestinal narrowing (pathologic or iatrogenic).
Adverse Reactions
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in more detail below and elsewhere in the labeling:
- •
- Hypoglycemia [see Warnings and Precautions (5.1)]
- •
- Hemolytic anemia [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials, 580 patients from 31 to 87 years of age received GLUCOTROL XL in doses from 5 mg to 60 mg in both controlled and open trials. The dosages above 20 mg are not recommended dosages. In these trials, approximately 180 patients were treated with GLUCOTROL XL for at least 6 months.
Table 1 summarizes the incidence of adverse reactions, other than hypoglycemia, that were reported in pooled double-blind, placebo-controlled trials in ≥3% of GLUCOTROL XL-treated patients and more commonly than in patients who received placebo.
| GLUCOTROL XL (%) (N=278) | Placebo (%) (N=69) | |
|---|---|---|
| Adverse Effect | ||
Dizziness | 6.8 | 5.8 |
Diarrhea | 5.4 | 0.0 |
Nervousness | 3.6 | 2.9 |
Tremor | 3.6 | 0.0 |
Flatulence | 3.2 | 1.4 |
Hypoglycemia
Of the 580 patients that received GLUCOTROL XL in clinical trials, 3.4% had hypoglycemia documented by a blood-glucose measurement <60 mg/dL and/or symptoms believed to be associated with hypoglycemia and 2.6% of patients discontinued for this reason. Hypoglycemia was not reported for any placebo patients.
Gastrointestinal Reactions
In clinical trials, the incidence of gastrointestinal (GI) side effects (nausea, vomiting, constipation, dyspepsia), occurred in less than 3% of GLUCOTROL XL-treated patients and were more common in GLUCOTROL XL-treated patients than those receiving placebo.
Dermatologic Reactions
In clinical trials, allergic skin reactions, i.e., urticaria occurred in less than 1.5% of treated patients and were more common in GLUCOTROL XL treated patients than those receiving placebo. These may be transient and may disappear despite continued use of glipizide XL; if skin reactions persist, the drug should be discontinued.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of GLUCOTROL XL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- •
- Abdominal pain
- •
- Cholestatic and hepatocellular forms of liver injury accompanied by jaundice
- •
- Leukopenia, agranulocytosis, thrombocytopenia, hemolytic anemia [see Warnings and Precautions (5.2)], aplastic anemia, pancytopenia
- •
- Hepatic porphyria and disulfiram-like reactions
- •
- Hyponatremia and the syndrome of inappropriate antidiuretic hormone (SIADH) secretion
- •
- Rash
- •
- There have been reports of gastrointestinal irritation and gastrointestinal bleeding with use of another drug with this non-dissolvable extended release formulation.
Drug Interactions
7 DRUG INTERACTIONS
7.1 Drugs Affecting Glucose Metabolism
A number of medications affect glucose metabolism and may require GLUCOTROL XL dose adjustment and close monitoring for hypoglycemia or worsening glycemic control.
The following are examples of medication that may increase the glucose lowering effect of GLUCOTROL XL, increase the susceptibility to and/or intensity of hypoglycemia: antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, propoxyphene, salicylates, somatostatin analogs (e.g., octreotide), sulfonamide antibiotics, nonsteroidal anti-inflammatory agents, chloramphenicol, probenecid, coumarins, voriconazole, H2 receptor antagonists, and quinolones. When these medications are administered to a patient receiving GLUCOTROL XL, monitor the patient closely for hypoglycemia. When these medications are discontinued from a patient receiving GLUCOTROL XL, monitor the patient closely for worsening glycemic control.
The following are examples of medication that may reduce the glucose-lowering effect of GLUCOTROL XL, leading to worsening glycemic control: atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), thyroid hormones, phenytoin, nicotinic acid, and calcium channel blocking drugs. When such drugs are administered to patients receiving GLUCOTROL XL, monitor the patients closely for worsening glycemic control. When these medications are discontinued from patients receiving GLUCOTROL XL, monitor the patient closely for hypoglycemia.
Alcohol, beta-blockers, clonidine, and reserpine may lead to either potentiation or weakening of the glucose-lowering effect. Increased frequency of monitoring may be required when GLUCOTROL XL is co-administered with these drugs.
The signs of hypoglycemia may be reduced or absent in patients taking sympatholytic drugs such as beta-blockers, clonidine, guanethidine, and reserpine. Increased frequency of monitoring may be required when GLUCOTROL XL is co-administered with these drugs.
7.2 Miconazole
Monitor patients closely for hypoglycemia when Glucotrol XL is co-administered with miconazole. A potential interaction between oral miconazole and oral hypoglycemic agents leading to severe hypoglycemia has been reported [see Clinical Phamacology (12.3)].
7.3 Fluconazole
Monitor patients closely for hypoglycemia when Glucotrol XL is co-administered with fluconazole. Concomitant treatment with fluconazole increases plasma concentrations of glipizide, which may lead to hypoglycemia [see Clinical Pharmacology (12.3)].
7.4 Colesevelam
GLUCOTROL XL should be administered at least 4 hours prior to the administration of colesevelam. Colesevelam can reduce the maximum plasma concentration and total exposure of glipizide when the two are coadministered [see Clinical Pharmacology (12.3)].
Use in Specific Populations
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from a small number of published studies and postmarketing experience with GLUCOTROL XL use in pregnancy over decades have not identified any drug associated risks for major birth defects, miscarriage, or adverse maternal outcomes. However, sulfonylureas (including glipizide) cross the placenta and have been associated with neonatal adverse reactions such as hypoglycemia. Therefore, GLUCOTROL XL should be discontinued at least two weeks before expected delivery (see Clinical Considerations). Poorly controlled diabetes in pregnancy is also associated with risks to the mother and fetus (see Clinical Considerations). In animal studies, there were no effects on embryofetal development following administration of glipizide to pregnant rats and rabbits during organogenesis at doses 833 times and 8 times the human dose based on body surface area, respectively. However, increased pup mortality was observed in rats administered glipizide from gestation day 15 throughout lactation at doses 2 times the maximum human dose based on body surface area (see Data).
The estimated background risk of major birth defects is 6–10% in women with pre-gestational diabetes with a HbA1c >7 and has been reported to be as high as 20–25% in women with HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Poorly-controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, miscarriage, preterm delivery, stillbirth, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Fetal/Neonatal Adverse Reactions
Neonates of women with gestational diabetes who are treated with sulfonylureas during pregnancy may be at increased risk for neonatal intensive care admission and may develop respiratory distress, hypoglycemia, birth injury, and be large for gestational age. Prolonged severe hypoglycemia, lasting 4–10 days, has been reported in neonates born to mothers receiving a sulfonylurea at the time of delivery and has been reported with the use of agents with a prolonged half-life. Observe newborns for symptoms of hypoglycemia and respiratory distress and manage accordingly.
Dose adjustments during pregnancy and the postpartum period
Due to reports of prolonged severe hypoglycemia in neonates born to mothers receiving a sulfonylurea at the time of delivery, GLUCOTROL XL should be discontinued at least two weeks before expected delivery (see Fetal/Neonatal Adverse Reactions).
Data
Animal Data
In teratology studies in rats and rabbits, pregnant animals received daily oral doses of glipizide during the period of organogenesis at doses up to 2000 mg/kg/day and 10 mg/kg/day (approximately 833 and 8 times the human dose based on body surface area), respectively. There were no adverse effects on embryo-fetal development at any of the doses tested. In a peri- and postnatal study in pregnant rats, there was a reduced number of pups born alive following administration of glipizide from gestation day 15 throughout lactation through weaning at doses ≥5 mg/kg/day (about 2 times the recommended maximum human dose based on body surface area).
8.2 Lactation
Risk Summary
Breastfed infants of lactating women using GLUCOTROL XL should be monitored for symptoms of hypoglycemia (see Clinical Considerations). Although glipizide was undetectable in human milk in one small clinical lactation study; this result is not conclusive because of the limitations of the assay used in the study. There are no data on the effects of glipizide on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for GLUCOTROL XL and any potential adverse effects on the breastfed child from GLUCOTROL XL or from the underlying maternal condition.
8.5 Geriatric Use
There were no overall differences in effectiveness or safety between younger and older patients, but greater sensitivity of some individuals cannot be ruled out. Elderly patients are particularly susceptible to the hypoglycemic action of anti-diabetic agents. Hypoglycemia may be difficult to recognize in these patients. Therefore, dosing should be conservative to avoid hypoglycemia [see Dosage and Administration (2.1), Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
There is no information regarding the effects of hepatic impairment on the disposition of glipizide. However, since glipizide is highly protein bound and hepatic biotransformation is the predominant route of elimination, the pharmacokinetics and/or pharmacodynamics of glipizide may be altered in patients with hepatic impairment. If hypoglycemia occurs in such patients, it may be prolonged and appropriate management should be instituted [see Dosage and Administration (2.1), Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
Overdosage
10 OVERDOSAGE
Overdosage of sulfonylureas including GLUCOTROL XL can produce severe hypoglycemia. Mild hypoglycemic symptoms without loss of consciousness or neurologic findings should be treated with oral glucose. Severe hypoglycemic reactions with coma, seizure, or other neurological impairment are medical emergencies requiring immediate treatment. The patient should be treated with glucagon or intravenous glucose. Patients should be closely monitored for a minimum of 24 to 48 hours since hypoglycemia may recur after apparent clinical recovery. Clearance of glipizide from plasma may be prolonged in persons with liver disease. Because of the extensive protein binding of glipizide, dialysis is unlikely to be of benefit.
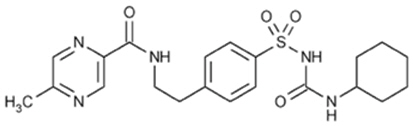
Description
11 DESCRIPTION
GLUCOTROL XL (glipizide) is an oral sulfonylurea.
The Chemical Abstracts name of glipizide is 1-cyclohexyl-3-[[p-[2-(5-methylpyrazinecarboxamido)ethyl] phenyl]sulfonyl]urea. The molecular formula is C21H27N5O4S; the molecular weight is 445.55; the structural formula is shown below:
Glipizide is a whitish, odorless powder with a pKa of 5.9. It is insoluble in water and alcohols, but soluble in 0.1 N NaOH; it is freely soluble in dimethylformamide.
Each tablet contains 2.75 mg glipizide to provide a 2.5 mg dose.
Each tablet contains 5.49 mg glipizide to provide a 5 mg dose.
Each tablet contains 10.98 mg glipizide to provide a 10 mg dose.
Inert ingredients in the 2.5 mg, 5 mg and 10 mg formulations are: polyethylene oxide, hypromellose, magnesium stearate, sodium chloride, red ferric oxide, cellulose acetate, polyethylene glycol, Opadry blue (OY-LS-20921)(2.5 mg tablets), Opadry white (YS-2-7063)(5 mg and 10 mg tablet) and Opacode Black Ink (S-1-17823).
System Components and Performance
GLUCOTROL XL Extended Release Tablet is similar in appearance to a conventional tablet. It consists, however, of an osmotically active drug core surrounded by a semipermeable membrane. The core itself is divided into two layers: an "active" layer containing the drug, and a "push" layer containing pharmacologically inert (but osmotically active) components. The membrane surrounding the tablet is permeable to water but not to drug or osmotic excipients. As water from the gastrointestinal tract enters the tablet, pressure increases in the osmotic layer and "pushes" against the drug layer, resulting in the release of drug through a small, laser-drilled orifice in the membrane on the drug side of the tablet.
The function of the GLUCOTROL XL Extended Release Tablet depends upon the existence of an osmotic gradient between the contents of the bi-layer core and fluid in the GI tract. The biologically inert components of the tablet remain intact during GI transit and are eliminated in the feces as an insoluble shell.
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Glipizide primarily lowers blood glucose by stimulating the release of insulin from the pancreas, an effect dependent upon functioning beta cells in the pancreatic islets. Sulfonylureas bind to the sulfonylurea receptor in the pancreatic beta-cell plasma membrane, leading to closure of the ATP-sensitive potassium channel, thereby stimulating the release of insulin.
12.2 Pharmacodynamics
The insulinotropic response to a meal is enhanced with GLUCOTROL XL administration in diabetic patients. The postprandial insulin and C-peptide responses continue to be enhanced after at least 6 months of treatment. In two randomized, double-blind, dose-response studies comprising a total of 347 patients, there was no significant increase in fasting insulin in all GLUCOTROL XL-treated patients combined compared to placebo, although minor elevations were observed at some doses.
In studies of GLUCOTROL XL in subjects with type 2 diabete mellitus, once daily administration produced reductions in hemoglobin A1c, fasting plasma glucose and postprandial glucose. The relationship between dose and reduction in hemoglobin A1c was not established, however subjects treated with 20 mg had a greater reduction in fasting plasma glucose compared to subjects treated with 5 mg.
12.3 Pharmacokinetics
Absorption
The absolute bioavailability of glipizide was 100% after single oral doses in patients with type 2 diabetes mellitus. Beginning 2 to 3 hours after administration of GLUCOTROL XL, plasma drug concentrations gradually rise reaching maximum concentrations within 6 to 12 hours after dosing. With subsequent once daily dosing of GLUCOTROL XL, plasma glipizide concentrations are maintained throughout the 24 hour dosing interval with less peak to trough fluctuation than that observed with twice daily dosing of immediate release glipizide.
The mean relative bioavailability of glipizide in 21 males with type 2 diabetes mellitus after administration of 20 mg GLUCOTROL XL, compared to immediate release Glucotrol (10 mg given twice daily), was 90% at steady-state. Steady-state plasma concentrations were achieved by at least the fifth day of dosing with GLUCOTROL XL in 21 males with type 2 diabetes mellitus and patients younger than 65 years. No accumulation of drug was observed in patients with type 2 diabetes mellitus during chronic dosing with GLUCOTROL XL.
Administration of GLUCOTROL XL with food has no effect on the 2 to 3 hour lag time in drug absorption. In a single dose, food effect study in 21 healthy male subjects, the administration of GLUCOTROL XL immediately before a high fat breakfast resulted in a 40% increase in the glipizide mean Cmax value, which was significant, but the effect on the AUC was not significant. There was no change in glucose response between the fed and fasting state. Markedly reduced GI retention times of the GLUCOTROL XL tablets over prolonged periods (e.g., short bowel syndrome) may influence the pharmacokinetic profile of the drug and potentially result in lower plasma concentrations.
In a multiple dose study in 26 males with type 2 diabetes mellitus, the pharmacokinetics of glipizide were linear with GLUCOTROL XL in that the plasma drug concentrations increased proportionately with dose. In a single dose study in 24 healthy subjects, four 5-mg, two 10-mg, and one 20-mg GLUCOTROL XL tablets were bioequivalent. In a separate single dose study in 36 healthy subjects, four 2.5-mg GLUCOTROL XL tablets were bioequivalent to one 10-mg GLUCOTROL XL tablet.
Distribution
The mean volume of distribution was approximately 10 liters after single intravenous doses in patients with type 2 diabetes mellitus. Glipizide is 98–99% bound to serum proteins, primarily to albumin.
Metabolism
The major metabolites of glipizide are products of aromatic hydroxylation and have no hypoglycemic activity. A minor metabolite, an acetylamino-ethyl benzene derivative, which accounts for less than 2% of a dose, is reported to have 1/10 to 1/3 as much hypoglycemic activity as the parent compound.
Elimination
Glipizide is eliminated primarily by hepatic biotransformation: less than 10% of a dose is excreted as unchanged drug in urine and feces; approximately 90% of a dose is excreted as biotransformation products in urine (80%) and feces (10%).
The mean total body clearance of glipizide was approximately 3 liters per hour after single intravenous doses in patients with type 2 diabetes mellitus. The mean terminal elimination half-life of glipizide ranged from 2 to 5 hours after single or multiple doses in patients with type 2 diabetes mellitus.
Specific Populations
Pediatric:
Studies characterizing the pharmacokinetics of glipizide in pediatric patients have not been performed.
Geriatric:
There were no differences in the pharmacokinetics of glipizide after single dose administration to older diabetic subjects compared to younger healthy subjects [see Use in Specific Populations (8.5)].
Renal Impairment:
The pharmacokinetics of glipizide has not been evaluated in patients with varying degree of renal impairment. Limited data indicates that glipizide biotransformation products may remain in circulation for a longer time in subjects with renal impairment than that seen in subjects with normal renal function.
Drug-drug Interactions
Miconazole
A potential interaction between oral miconazole and oral glipizide leading to severe hypoglycemia has been reported. Whether this interaction also occurs with the intravenous, topical, or vaginal preparations of miconazole is not known [see Drug Interactions (7.2)].
Fluconazole
Concomitant treatment with fluconazole increases plasma concentrations of glipizide. The effect of concomitant administration of Diflucan (fluconazole) and Glucotrol has been demonstrated in a placebo controlled crossover study in healthy volunteers. All subjects received Glucotrol alone and following treatment with 100 mg of Diflucan as a single daily oral dose for 7 days. The mean percentage increase in the glipizide AUC after fluconazole administration was 56.9% (range: 35 to 81%) [see Drug Interactions (7.3)].
Colesevelam
Colesevelam can reduce the maximum plasma concentration and total exposure of glipizide when the two are coadministered. In studies assessing the effect of colesevelam on the pharmacokinetics of glipizide ER in healthy volunteers, reductions in glipizide AUC0–∞ and Cmax of 12% and 13%, respectively were observed when colesevelam was coadministered with glipizide ER. When glipizide ER was administered 4 hours prior to colesevelam, there was no significant change in glipizide AUC0–∞ or Cmax, -4% and 0%, respectively [see Drug Interactions (7.4)].
Nonclinical Toxicology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A twenty month study in rats and an eighteen month study in mice at doses up to 75 times the maximum human dose revealed no evidence of drug-related carcinogenicity. Bacterial and in vivo mutagenicity tests were uniformly negative. Studies in rats of both sexes at doses up to 20 times the human dose based on body surface area, showed no effects on fertility.
References
How Supplied/Storage and Handling
16 HOW SUPPLIED/STORAGE AND HANDLING
GLUCOTROL XL (glipizide) Extended Release Tablets are supplied to provide 2.5 mg, 5 mg, and 10 mg round, biconvex tablets and imprinted with black ink as follows:
| Tablet Strength | Tablet Color/Shape | Tablet Markings | Package Size | NDC Code |
|---|---|---|---|---|
2.5 mg | Blue | imprinted with "GXL 2.5" on one side | Bottles of 30 | NDC 0049-0170-01 |
5 mg | White | imprinted with "GXL 5" on one side | Bottles of 100 | NDC 0049-0174-02 |
10 mg | White | imprinted with "GXL 10" on one side | Bottles of 100 | NDC 0049-0178-07 |
Medication Guide
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform patients of the potential adverse reactions of GLUCOTROL XL including hypoglycemia. Explain the risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development to patients and responsible family members. Also inform patients about the importance of adhering to dietary instructions, of a regular exercise program, and of regular testing of glycemic control.
Inform patients that GLUCOTROL XL should be swallowed whole. Inform patients that they should not chew, divide or crush tablets and they may occasionally notice in their stool something that looks like a tablet. In the GLUCOTROL XL tablet, the medication is contained within a non-dissolvable shell that has been specially designed to slowly release the drug so the body can absorb it.
Pregnancy
Advise females of reproductive potential to inform their prescriber of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding women taking GLUCOTROL XL to monitor breastfed infants for signs of hypoglycemia (e.g., jitters, cyanosis, hypothermia, excessive sleepiness, poor feeding, seizures) [see Use in Specific Populations (8.2)].
This Patient Information has been approved by the U.S. Food and Drug Administration | Revised 08/2023 | |
PATIENT INFORMATION | ||
What is GLUCOTROL XL?
It is not known if GLUCOTROL XL is safe and effective in children under 18 years of age. | ||
Who Should Not Take GLUCOTROL XL?
| ||
What should I tell my doctor before taking GLUCOTROL XL?
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | ||
How should I take GLUCOTROL XL?
| ||
What should I avoid while taking GLUCOTROL XL?
| ||
What are the possible side effects of GLUCOTROL XL?
| ||
|
| |
If you have signs or symptoms of low blood sugar, eat or drink something with sugar in it right away. If you do not feel better or your blood sugar level does not go up, call your healthcare provider or go to the nearest emergency room. | ||
How to store GLUCOTROL XL?
Keep GLUCOTROL XL and all medicines out of reach of children. | ||
General information about the safe and effective use of GLUCOTROL XL. | ||
What are the ingredients in GLUCOTROL XL? | ||

| ||
Other
This product's label may have been updated. For full prescribing information, please visit www.pfizer.com.
LAB-0113-17.0
What is GLUCOTROL XL?
What is GLUCOTROL XL?
- •
- GLUCOTROL XL is a prescription medicine you take by mouth used along with diet and exercise to lower blood sugar in adults with type 2 diabetes mellitus.
- •
- GLUCOTROL XL is not for people with type 1 diabetes or people with diabetic ketoacidosis.
It is not known if GLUCOTROL XL is safe and effective in children under 18 years of age.
Who Should Not Take GLUCOTROL XL?
Who Should Not Take GLUCOTROL XL?
Do not use GLUCOTROL XL if you:- •
- have a condition called diabetic ketoacidosis
- •
- have ever had an allergic reaction to glipizide or any of the other ingredients in GLUCOTROL XL. See the end of this Patient Information for a complete list of ingredients in GLUCOTROL XL.
What should I tell my doctor before taking GLUCOTROL XL?
What should I tell my doctor before taking GLUCOTROL XL?
Before you take GLUCOTROL XL, tell your healthcare provider if you:- •
- Have ever had a condition called diabetic ketoacidosis
- •
- Have kidney or liver problems
- •
- Have had a blockage or narrowing of your intestines due to illness or past surgery
- •
- Have chronic (continuing) diarrhea
- •
- Have glucose-6-phosphate dehydrogenase (G6PD) deficiency. This condition usually runs in families. People with G6PD deficiency who take GLUCOTROL XL may develop hemolytic anemia (fast breakdown of red blood cells).
- •
- Are pregnant or might be pregnant. It is not known if GLUCOTROL XL will harm your unborn baby. If you are pregnant, talk to your healthcare provider about the best way to control your blood sugar while you are pregnant. You should not take GLUCOTROL XL during the last two weeks of pregnancy.
- •
- Are breastfeeding or plan to breastfeed. It is not known if GLUCOTROL XL passes into your breast milk. You and your healthcare provider should decide the best way to feed your baby during treatment with GLUCOTROL XL.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
GLUCOTROL XL may affect the way other medicines work, and other medicines may affect how GLUCOTROL XL works.
Some medicines can affect how well GLUCOTROL XL works or may affect you blood sugar level.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take GLUCOTROL XL?
How should I take GLUCOTROL XL?
- •
- Take GLUCOTROL XL exactly as your healthcare provider tells you to take it.
- •
- Your healthcare provider will tell you how much GLUCOTROL XL to take and when to take it.
- •
- Take GLUCOTROL XL by mouth, 1 time each day with breakfast or your first meal of the day.
- •
- Each GLUCOTROL XL tablet will release the medicine slowly over 24 hours. This is why you take it only 1 time each day.
- •
- Swallow the GLUCOTROL XL whole. Do not break, crush, dissolve, chew, or cut the tablet in half. This will damage the tablet and release too much medicine into your body at one time.
- •
- When you take GLUCOTROL XL you may see something in your stool that looks like a tablet. This is the empty shell from the tablet. It is normal for the empty shell to pass with your bowel movement after medicine has been absorbed by your body.
- •
- It is important to take GLUCOTROL XL every day to help keep your blood sugar level under good control. Your healthcare provider may change your dose depending on your blood sugar test results. If your blood sugar level is not under control, call your healthcare provider. Do not change your dose unless your healthcare provider tells you to.
- •
- If you take too much GLUCOTROL XL, call your healthcare provider or go to the nearest emergency room right away.
Your healthcare provider may tell you to take GLUCOTROL XL with other diabetes medicines. Low blood sugar can happen more often when GLUCOTROL XL is taken with other diabetes medicines. See "What are the possible side effects of GLUCOTROL XL?" - •
- Check your blood sugar as your healthcare provider tells you to.
- •
- Stay on your prescribed diet and exercise program while taking GLUCOTROL XL.
What should I avoid while taking GLUCOTROL XL?
What should I avoid while taking GLUCOTROL XL?
- •
- Do not drink alcohol while taking GLUCOTROL XL. It can increase your chances of getting serious side effects.
- •
- Do not drive, operate machinery, or do other dangerous activities until you know how GLUCOTROL XL affects you.
What are the possible side effects of GLUCOTROL XL?
What are the possible side effects of GLUCOTROL XL?
GLUCOTROL XL can cause serious side effects, including:- •
- Low blood sugar. GLUCOTROL XL may cause low blood sugar. Signs and symptoms of low blood sugar may include:
- •
- a cold clammy feeling
- •
- unusual sweating
- •
- dizziness
- •
- weakness
- •
- trembling
- •
- shakiness
- •
- hunger
- •
- fast heartbeat
- •
- headache
- •
- blurred vision
- •
- slurred speech
- •
- tingling in the lips or hands
If you have signs or symptoms of low blood sugar, eat or drink something with sugar in it right away. If you do not feel better or your blood sugar level does not go up, call your healthcare provider or go to the nearest emergency room.
The most common side effects of GLUCOTROL XL include: dizziness, diarrhea, nervousness, tremor, and gas.
These are not all the possible side effects of GLUCOTROL XL. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How to store GLUCOTROL XL?
How to store GLUCOTROL XL?
- •
- Store GLUCOTROL XL at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Store GLUCOTROL XL in a dry place, in its original container.
Keep GLUCOTROL XL and all medicines out of reach of children.
General information about the safe and effective use of GLUCOTROL XL.
General information about the safe and effective use of GLUCOTROL XL.
www.pfizer.comWhat are the ingredients in GLUCOTROL XL?
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform patients of the potential adverse reactions of GLUCOTROL XL including hypoglycemia. Explain the risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development to patients and responsible family members. Also inform patients about the importance of adhering to dietary instructions, of a regular exercise program, and of regular testing of glycemic control.
Inform patients that GLUCOTROL XL should be swallowed whole. Inform patients that they should not chew, divide or crush tablets and they may occasionally notice in their stool something that looks like a tablet. In the GLUCOTROL XL tablet, the medication is contained within a non-dissolvable shell that has been specially designed to slowly release the drug so the body can absorb it.
Pregnancy
Advise females of reproductive potential to inform their prescriber of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding women taking GLUCOTROL XL to monitor breastfed infants for signs of hypoglycemia (e.g., jitters, cyanosis, hypothermia, excessive sleepiness, poor feeding, seizures) [see Use in Specific Populations (8.2)].
This Patient Information has been approved by the U.S. Food and Drug Administration | Revised 08/2023 | |
PATIENT INFORMATION | ||
What is GLUCOTROL XL?
It is not known if GLUCOTROL XL is safe and effective in children under 18 years of age. | ||
Who Should Not Take GLUCOTROL XL?
| ||
What should I tell my doctor before taking GLUCOTROL XL?
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | ||
How should I take GLUCOTROL XL?
| ||
What should I avoid while taking GLUCOTROL XL?
| ||
What are the possible side effects of GLUCOTROL XL?
| ||
|
| |
If you have signs or symptoms of low blood sugar, eat or drink something with sugar in it right away. If you do not feel better or your blood sugar level does not go up, call your healthcare provider or go to the nearest emergency room. | ||
How to store GLUCOTROL XL?
Keep GLUCOTROL XL and all medicines out of reach of children. | ||
General information about the safe and effective use of GLUCOTROL XL. | ||
What are the ingredients in GLUCOTROL XL? | ||

| ||
Resources
Didn’t find what you were looking for?
Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this product, click the link below to submit your information: Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer product, please call Pfizer Medical Information at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800)-332-1088.